Abstract
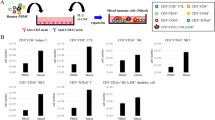
Genetically modified dendritic cells (DC) are increasingly used in vitro to activate cytotoxic T lymphocyte (CTL) immune responses. Because T cell activation protocols consist of multiple restimulation cycles of peripheral blood lymphocytes with antigen-loaded mature DC, continuous generation of DC is needed throughout the experiment. Therefore, cryopreservation of DC loaded with antigen is a valuable alternative for weekly generation and modification of DC. Recently, we described an antigen loading method for DC based on electroporation of defined tumor antigen mRNA. In this study, we demonstrate that mRNA-electroporated DC can efficiently be prepared for cryopreservation. Using an optimized maturation and freezing protocol after mRNA electroporation, we obtained high transgene-expressing viable mature DC. In addition, we showed that these modified cryopreserved DC retain stimulatory capacity in an influenza model system. Therefore, cryopreservation of mature mRNA-electroporated DC is a useful method for continuous availability of antigen-loaded DC throughout T cell activation experiments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banchereau J, Steinman RM . Dendritic cells and the control of immunity Nature 1998 392: 245–252
Van Tendeloo V, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN . Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells Blood 2001 98: 49–56
Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E . Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA Nat Biotechnol 1998 16: 364–369
Strobel I, Berchtold S, Gotze A, Schulze U, Schuler G, Steinkasserer A . Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes Gene Ther 2000 7: 2028–2035
Schattenberg D, Schott M, Reindl G, Krueger T, Tschoepe D, Feldkamp J, Scherbaum WA, Seissler J . Response of human monocyte-derived dendritic cells to immunostimulatory DNA Eur J Immunol 2000 30: 2824–2831
Gilboa E . The makings of a tumor rejection antigen Immunity 1999 11: 263–270
Makino M, Baba M . A cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells Scand J Immunol 1997 45: 618–622
Lewalle P, Rouas R, Lehmann F, Martiat P . Freezing of dendritic cells, generated from cryopreserved leukaphereses, does not influence their ability to induce antigen-specific immune responses or functionally react to maturation stimuli J Immunol Methods 2000 240: 69–78
Feuerstein B, Berger TG, Maczek C, Röder C, Schreiner D, Hirsch U, Haendle I, Leisgang W, Glaser A, Kuss O, Diepgen TL, Schuler G, Schuler-Thurner B . A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use J Immunol Methods 2000 245: 15–29
Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G . Proliferating dendritic cell progenitors in human blood J Exp Med 1994 180: 83–93
Van Tendeloo VFI, Snoeck H-W, Lardon F, Vanham GLEE, Nijs G, Lenjou M, Hendricks L, Van Broeckhoven C, Moulijn A, Rodrigus I, Verdonk P, Van Bockstaele DR, Berneman ZN . Non-viral transfection of distinct types of human dendritic cells: high-efficiency gene transfer by electroporation into hematopoietic progenitor- but not monocyte-derived dendritic cells Gene Ther 1998 5: 700–707
Larsson M, Messmer D, Somersan S, Fonteneau JF, Donahoe SM, Lee M, Dunbar PR, Cerundolo V, Julkunun I, Nixon DF, Bhardwaj N . Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells J Immunol 2000 165: 1182–1190
Thornburg C, Boczkowski D, Gilboa E, Nair SK . Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus E6 and E7 RNA: implications for cervical cancer immunotherapy J Immunother 2000 23: 412–418
Acknowledgements
This work was supported by grant Nos 3.0109.96, G.0157.99 and G.0313.01 of the Fund for Scientific Research – Flanders, Belgium (FWO), by grants of the Scientific Committee of the Fortis Bank (FB)-financed Cancer Research and of the Belgian Federation against Cancer (BFK). PP, NC, AVD are holders of a PhD fellowship from the Flemish Institute for Science and Technology (IWT). VFIVT is a postdoctoral fellow of the FWO.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ponsaerts, P., Van Tendeloo, V., Cools, N. et al. mRNA-electroporated mature dendritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreservation. Leukemia 16, 1324–1330 (2002). https://doi.org/10.1038/sj.leu.2402511
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402511
Keywords
This article is cited by
-
Efficient large volume electroporation of dendritic cells through micrometer scale manipulation of flow in a disposable polymer chip
Biomedical Microdevices (2011)
-
A Short Pulse of IL-4 Delivered by DCs Electroporated With Modified mRNA Can Both Prevent and Treat Autoimmune Diabetes in NOD Mice
Molecular Therapy (2010)
-
Polyelectrolyte Capsules-containing HIV-1 p24 and Poly I:C Modulate Dendritic Cells to Stimulate HIV-1-specific Immune Responses
Molecular Therapy (2010)
-
The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells
Cancer Immunology, Immunotherapy (2010)
-
Efficient gene transfer in CLL by mRNA electroporation
Leukemia (2008)