Abstract
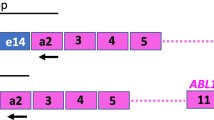
The AML1/ETO fusion transcript can be detected by reverse transcription polymerase chain reaction (RT-PCR) in patients with t(8;21)-associated acute myeloid leukemia (AML) in long-term complete remission (CR). Quantitation of the amount of the fusion transcript during CR may therefore be more predictive of cure or relapse than a simple qualitative assessment. Real Time PCR, a fluorometric-based technique, allows simple and rapid quantitation of a target sequence during the extension phase of PCR amplification, in contrast to end-point quantitative methods. Six patients with t(8;21)(q22;q22) AML, who achieved CR were studied by Real Time RT-PCR at different time intervals following diagnosis and high-dose cytarabine and anthracycline-based induction therapy. Five patients had a diagnostic bone marrow (BM) sample available for molecular analysis. Each patient showed ⩾103 copies of the AML1/ETO fusion transcript at diagnosis, and each showed a 2- to 4-log decrease in copy number following successful induction chemotherapy. This is comparable to the log-fold reduction in leukemic blasts that is thought to occur in patients successfully cytoreduced into CR by induction chemotherapy. The sixth patient showed a relatively high copy number immediately following successful remission induction chemotherapy, which continued to increase during early CR and was later followed by relapse. Real Time RT-PCR appears to offer advantages over previously used quantitative RT-PCR methods by providing absolute quantitation of the target sequence, expanding the dynamic range of quantitation to over six orders of magnitude, eliminating the post-PCR processing, and reducing labor and carryover contamination. These features make this an attractive method to prospectively evaluate the prognostic value of AML1/ETO fusion transcript quantitation in a larger patient population with t(8;21)(q22;q22) AML in CR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marcucci, G., Livak, K., Bi, W. et al. Detection of minimal residual disease in patients with AML1/ETO-associated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay. Leukemia 12, 1482–1489 (1998). https://doi.org/10.1038/sj.leu.2401128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401128
Keywords
This article is cited by
-
Identification of molecular and cytogenetic risk factors for unfavorable core-binding factor-positive adult AML with post-remission treatment outcome analysis including transplantation
Bone Marrow Transplantation (2014)
-
Monitoring of minimal residual disease in acute myeloid leukemia with t(8;21)(q22;q22)
International Journal of Hematology (2013)
-
Clinical implications of molecular genetic aberrations in acute myeloid leukemia
Journal of Cancer Research and Clinical Oncology (2009)
-
Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)]
Leukemia (2006)
-
Prognostic value of real-time quantitative PCR (RQ-PCR) in AML with t(8;21)
Leukemia (2005)