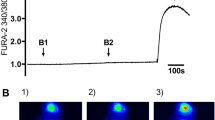
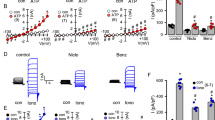
Abstract
The mucociliary system is responsible for clearing inhaled particles and pathogens from the airways. This important task is performed by the beating of cilia and the consequent movement of mucus from the lungs to the upper airways1,2. Because ciliary motility is enhanced by elevated intracellular calcium concentrations, inhibition of calcium influx could lead to disease by jeopardizing mucociliary clearance. Several hormones and neurotransmitters stimulate ciliary motility, one of the most potent of which is extracellular ATP (ATPo)1, which acts by releasing calcium ions from internal stores and by activating calcium influx3,4,5. Here we show that, in airway ciliated cells, extracellular sodium ions (Nao+) specifically and competitively inhibit an ATPo-gated channel that is permeable to calcium ions, and thereby attenuate ATPo-induced ciliary motility. Our finding points to a physiological role for Nao+ in ciliary function, and indicates that mucociliary clearance might be improved in respiratory disorders such as chronic bronchitis and cystic fibrosis by decreasing the sodium concentration of the airway surface fluid in which the cilia are bathed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wanner, A., Salathé, M. & O'riordan, T. G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 154, 1868–1902 (1996).
Matsui, H., Randell, S. H., Peretti, S. W., Davis, C. W. & Boucher, R. C. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J. Clin. Invest. 102, 1125–1131 (1998).
Paradiso, A. M., Mason, S. J., Lazarowski, E. R. & Boucher, R. C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature 377, 643–646 (1995).
Korngreen, A. & Priel, Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J. Physiol. (Lond.) 497, 53–66 (1996).
Uzlaner, N. & Priel, Z. Interplay between the NO pathway and elevated [Ca2+]ienhances ciliary activity in rabbit trachea. J. Physiol. (Lond.) 516, 179–190 (1999).
Korngreen, A., Ma, W., Pirel, Z. & Silberberg, S. D. Extracellular ATP directly gates a cation-selective channel in rabbit airway ciliated epithelial cells. J. Physiol. (Lond.) 508, 703–720 (1998).
Silberberg, A. On mucociliary transport. Biorheology 27, 295–307 (1990).
Smith, J. J., Travis, S. M., Greenberg, E. P. & Welsh, M. J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85, 229–236 (1996).
Goldman, M. J. et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88, 553–560 (1997).
Bals, R. et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Invest. 102, 874–880 (1998).
Joris, L., Dab, I. & Quinton, P. M. Elemental composition of human airway surface fluid in healthy and diseased airways. Am. Rev. Respir. Dis. 148, 1633–1637 (1993).
Knowles, M. R. et al. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J. Clin. Invest. 100, 2588–2595 (1997).
Hull, J., Skinner, W., Robertson, C. & Phelan, P. Elemental content of airway surface liquid from infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 157, 10–14 (1998).
Matsui, H. et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airway disease. Cell 95, 1005–1015 (1999).
Zabner, J., Smith, J. J., Karp, P. H., Widdicombe, J. H. & Welsh, M. J. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell 2, 397–403 (1998).
Wine, J. J. The genesis of cystic fibrosis lung disease. J. Clin. Invest. 103, 309–312 (1999).
Daviskas, E. et al. Inhalation of dry-powder mannitol increases mucociliary clearance. Eur. Respir. J. 10, 2449–2454 (1997).
Wills, P. J., Hall, R. L. Chan, W. M. & Cole, P. J. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus-depleted bovine trachea. J. Clin. Invest. 99, 9–13 (1997).
Pavia, D., Thomson, M. L. & Clarke, S. W. Enhanced clearance of secretions from the human lung after the administration of hypertonic saline aerosol. Am. Rev. Respir. Dis. 117, 199–203 (1978).
Daviskas, E. et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur. Respir. J. 9, 725–732 (1996).
Eng, P. A. et al. Short-term efficacy of ultrasonically nebulized hypertonic saline in cystic fibrosis. Pediatr. Pulmonol. 21, 77–83 (1996).
Robinson, M. et al. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 153, 1503–1509 (1996).
Robinson, M. et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 52, 900–903 (1997).
Boitano, S., Woodruff, M. L. & Dirksen, E. R. Reduction of extracellular Na+ causes a release of Ca2+ from internal stores in airway epithelial cells. Am. J. Physiol. 272, L1189–L1197 (1997).
Acknowledgements
We thank G. David, Z. Gill, M. J. Gutnick, K. L. Magleby, B. Sakmann and D. S. Weiss for comments and suggestions on the manuscript, and Y. Rodrig for providing freshly dissociated pig airway ciliated cells. This work was supported by The Israel Science Foundation founded by the Israel Academy of Sciences and Humanities — Dorot Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, W., Korngreen, A., Uzlaner, N. et al. Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature 400, 894–897 (1999). https://doi.org/10.1038/23743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23743
This article is cited by
-
Influenza A virus enhances ciliary activity and mucociliary clearance via TLR3 in airway epithelium
Respiratory Research (2020)
-
Prospective, randomized, controlled, open-label study to compare efficacy of a mineral-rich solution vs normal saline after complete ethmoidectomy
European Archives of Oto-Rhino-Laryngology (2019)
-
The role of osmolality in saline fluid nebulization after tracheostomy: time for changing?
BMC Pulmonary Medicine (2016)
-
Methods to measure and analyze ciliary beat activity: Ca2+ influx-mediated cilia mechanosensitivity
Pflügers Archiv - European Journal of Physiology (2012)
-
The touching story of purinergic signaling in epithelial and endothelial cells
Purinergic Signalling (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.