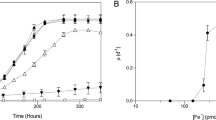
Abstract
Dissolved-iron availability plays a critical role in controlling phytoplankton growth in the oceans1,2. The dissolved iron is overwhelmingly (∼99%) bound to organic ligands with a very high affinity for iron3,4,5, but the origin, chemical identity and biological availability of this organically complexed Fe is largely unknown6. The release into sea water of complexes that strongly chelate iron could result from the inducible iron-uptake systems of prokaryotes (siderophore complexes)7,8,9 or by processes such as zooplankton-mediated degradation and release of intracellular material (porphyrin complexes). Here we compare the uptake of siderophore- and porphyrin-complexed 55Fe by phytoplankton, using both cultured organisms and natural assemblages. Eukaryotic phytoplankton efficiently assimilate porphyrin-complexed iron, but this iron source is relatively unavailable to prokaryotic picoplankton (cyanobacteria). In contrast, iron bound to a variety of siderophores is relatively more available to cyanobacteria than to eukaryotes, suggesting that the two plankton groups exhibit fundamentally different iron-uptake strategies. Prokaryotes utilize iron complexed to either endogenous7,8,9 or exogenous siderophores9, whereas eukaryotes may rely on a ferrireductase system10,11 that preferentially accesses iron chelated by tetradentate porphyrins, rather than by hexadentate siderophores. Competition between prokaryotes and eukaryotes for organically-bound iron may therefore depend on the chemical nature of available iron complexes, with consequences for ecological niche separation, plankton community size-structure and carbon export in low-iron waters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Coale, K. H. et al. Amassive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383, 495–501 (1996).
Hutchins, D. A. & Bruland, K. W. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393, 561–564 (1998).
Wu, J. & Luther, G. W. Complexation of Fe(III) by natural organic ligands in the Northwest Atlantic Ocean by a competitive ligand method and a kinetic approach. Mar. Chem. 50, 159–177 (1995).
Rue, E. L. & Bruland, K. W. Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 50, 117–138 (1995).
Witter, A. E. & Luther, G. W. Variation in Fe-organic complexation with depth in the Northwestern Atlantic Ocean as determined using a kinetic approach. Mar. Chem. 62, 241–258 (1998).
Hutchins, D. A. in Progress in Phycological Research Vol. 11(eds Chapman, D. & Round, F.) 1–49 (Biopress, Bristol, 1995).
Wilhelm, S. W. Ecology of iron-limited cyanobacteria: a review of physiological responses and implications for aquatic systems. Aquat. Microbial Ecol. 9, 295–303 (1995).
Butler, A. Acquisition and utilization of transition metal ions by marine organisms. Science 281, 207–210 (1998).
Granger, J. & Price, N. M. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol. Oceanogr. 44, 541–555 (1999).
Jones, G. J., Palenik, B. P. & Morel, F. M. M. Trace metal reduction by phytoplankton: the role of plasmalemma redox enzymes. J. Phycol. 23, 237–244 (1987).
Weger, H. G. Ferric and cupric reductase activities in the green alga Chalmydomonas reinhardtii: experiments using iron-limited chemostats. Planta 207, 377–384 (1999).
Reid, R. T., Live, D. H., Faulkner, D. J. & Butler, A. Asiderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature 366, 455–458 (1993).
Hutchins, D. A. & Bruland, K. W. Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey. Mar. Ecol. Prog. Ser. 110, 259–269 (1994).
Gobler, C. J. et al. Elemental release and bioavailability following viral lysis of a marine chrysophyte. Limnol. Oceanogr. 42, 1492–1504 (1997).
Strom, S. L. Production of phaeopigments by marine protozoa: results of laboratory experiments analyzed by HPLC. Deep Sea Res. I 40, 57–80 (1993).
Head, E. J. H. & Harris, L. R. Feeding selectivity by copepods grazing on natural mixtures of phytoplankton determined by HPLC analysis of pigments. Mar. Ecol. Prog. Ser. 110, 75–83 (1994).
McCarthy, M., Pratum, T., Hedges, J. & Benner, R. Chemical composition of dissolved organic nitrogen in the sea. Nature 390, 150–154 (1997).
Hudson, R. J. M. & Morel, F. M. M. Iron transport in marine phytoplankton: Kinetics of cellular and medium coordination reactions. Limnol. Oceanogr. 35, 1002–1020 (1990).
Bruland, K. W., Donat, J. R. & Hutchins, D. A. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol. Oceanogr. 36, 1555–1577 (1991).
Rich, H. R. & Morel, F. M. M. Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 35, 1555–1577 (1991).
Robinson, N. J., Procter, C. M., Connolly, E. L. & Guerinot, M. L. Aferric-chelate reductase for iron uptake from soils. Nature 397, 694–697 (1999).
Eide, D. Molecular biology of iron and zinc uptake in eukaryotes. Curr. Opin. Cell Biol. 9, 573–577 (1997).
Weinberg, E. D. Cellular regulation of iron assimilation. Q. Rev. Biol. 64, 261–290 (1989).
Brand, L. E. Minimum iron requirements of marine phytoplankton and the implications for the biogeochemical control of new production. Limnol. Oceanogr. 36, 1756–1771 (1991).
Sunda, W. G. & Huntsman, S. A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 50, 189–206 (1995).
Boyd, P. W. & Newton, P. P. Does planktonic community structure determine downward particulate organic carbon flux in different oceanic provinces? Deep-Sea Res. I 46, 63–91 (1999).
Lewis, B. L. et al. Voltammetric estimation of iron(III) thermodynamic stability constants for catecholate siderophores isolated from marine bacteria and cyanobacteria. Mar. Chem. 50, 179–188 (1995).
Hutchins, D. A., DiTullio, G. R., Zhang, Y. & Bruland, K. W. An iron limitation mosaic in the California upwelling regime. Limnol. Oceanogr. 43, 1037–1054 (1998).
Hudson, R. J. M. & Morel, F. M. M. Distinguishing between extra- and intracellular iron in marine phytoplankton. Limnol. Oceanogr. 34, 1113–1120 (1989).
Schmidt, M. A. & Hutchins, D. A. Size-fractionated biological iron and carbon uptake along a coastal to offshore transect in the NE Pacific. Deep-Sea Res. II 46, 1999).
Acknowledgements
We thank D. Kirchman, W. Sunda, S. Wilhelm, Y. Zhang and the captain and crew of the RV Cape Henlopen. This work was supported by the US NSF Biological and Chemical Oceanography (D.A.H., A.E.W. and G.W.L.), NIH, and California Sea Grant (A.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hutchins, D., Witter, A., Butler, A. et al. Competition among marine phytoplankton for different chelated iron species. Nature 400, 858–861 (1999). https://doi.org/10.1038/23680
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23680
This article is cited by
-
Short-term acidification promotes diverse iron acquisition and conservation mechanisms in upwelling-associated phytoplankton
Nature Communications (2023)
-
Isolation of dissolved organic matter from aqueous solution by precipitation with FeCl3: mechanisms and significance in environmental perspectives
Scientific Reports (2023)
-
Infect while the iron is scarce: nutrient-explicit phage-bacteria games
Theoretical Ecology (2021)
-
Excess labile carbon promotes the expression of virulence factors in coral reef bacterioplankton
The ISME Journal (2018)
-
Laboratory culture experiments to study the effect of lignite humic acid fractions on iron solubility and iron uptake rates in phytoplankton
Journal of Applied Phycology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.