Abstract
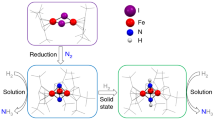
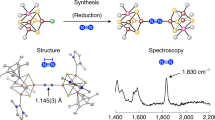
THE recent synthesis of a wide variety of transition metal complexes of molecular nitrogen (dinitrogen) has underlined the strong possibility that formation of a molybdenum—or iron—dinitrogen complex is an initial step in biological nitrogen fixation. Furthermore, complexes have been synthesized in which the dinitrogen ligand bridges two transition metals. These exhibit a substantial reduction in the N–N stretching frequency, ν(N2), and it may well be that dinitrogen is activated by bridging iron and molybdenum in the metallo-enzyme itself1. Specifically, Chatt has suggested that dinitrogen is probably taken up initially at the iron site, to form a dinitrogen complex of rather low ν(N2), which is lowered still further by the attachment of the molybdenum to the free end of the dinitrogen ligand, and thereby made more susceptible to facile reduction. An extension of this hypothesis is that of R. W. F. Hardy and E. Knight who suggest (personal communication) that one metal may be present as a hydride, in which case the following intermediate would result from insertion of the dinitrogen complex into the metal hydride bond: 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chatt, J., Dilworth, J. R., Richards, R. L., and Saunders, J. R., Nature, 224, 1201 (1969).
Parshall, G. W., J. Amer. Chem. Soc., 89, 1822 (1967).
Toniolo, L., and Eisenberg, R., Chem. Commun., 455 (1971).
Einstein, F. W. B., Gilchrist, A. B., Rayner-Canham, G. W., and Sutton, D., J. Amer. Chem. Soc., 93, 1826 (1971).
Shustorovich, E. M., J. Struct. Chem. (USSR), 11, 154 (1970).
Parshall, G. W., Accounts Chem. Res., 3, 139 (1970).
Cheney, A. J., Mann, B. E., Shaw, B. L., and Slade, R. M., Chem. Commun., 1176 (1970).
Cope, A. C., and Siekman, R. W., J. Amer. Chem. Soc., 87, 3272 (1965).
Owsley, D. C., and Helmkamp, G. K., J. Amer. Chem. Soc. 89, 4558 (1967).
Ellesmann, J., Poersch, F., Kunstmann, R., and Kamolowsky, R., Angew. Chem., Intern. Ed., 8, 203 (1969).
Newton, W. E., Corben, J. L., Schneider, P. W., and Bulen, W. A., J. Amer. Chem. Soc., 93, 268 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GILCHRIST, A., RAYNER-CANHAM, G. & SUTTON, D. Transition Metal Complexes of Diazonium Salts as Models for Nitrogenase. Nature 235, 42–44 (1972). https://doi.org/10.1038/235042a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/235042a0
This article is cited by
-
Zur katalytischen Reduktion aromatischer Stickstoffverbindungen und Diazene in Gegenwart von �bergangsmetallverbindungen
Monatshefte f�r Chemie (1978)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.