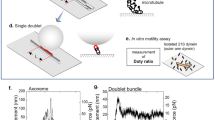
Abstract
Axonemal dyneins are force-generating ATPases that produce movement of eukaryotic cilia and flagella1,2. Several studies indicate that inner-arm dyneins mainly produce bending moments in flagella3,4 and that these motors have inherent oscillations in force and motility5,6,7,8. Processive motors such as kinesins have high duty ratios of attached to total ATPase cycle (attached plus detached) times9 compared to sliding motors such as myosin10. Here we provide evidence that subspecies-c, a single-headed axonemainner-arm dynein, is processive but has a low duty ratio. Ultrastructurally it is similar to other dyneins11,12,13,14, with a single globular head, long stem and a slender stalk that attaches to microtubules. In vitro studies of microtubules sliding over surfaces coated with subspecies-c at low densities (measured by single-molecule fluorescence) show that a single molecule is sufficient to move a microtubule more than 1 µm at 0.7 µm s−1. When many motors interact the velocity is 5.1 µm s−1, fitting a duty ratio of 0.14. Using optical trap nanometry, we show that beads carrying a single subspecies-c motor move processively along the microtubules in 8-nm steps but slip backwards under high loads. These results indicate that dynein subspecies-c functions in a very different way from conventional motor proteins, and has properties that could produce self-oscillation in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gibbons, I. R. Cilia and flagella of eukaryotes. J. Cell Biol. 91, 107s–124s (1981).
Holzbaur, E. L. F. & Vallee, R. B. Dyneins: Molecular structure and cellular function. Annu. Rev. Cell Biol. 10, 339–372 (1994).
Kamiya, R. Exploring the function of inner and outer dynein arms with Chlamydomonas mutants. Cell Motil. Cytoskeleton 32, 98–102 (1995).
Brokaw, C. J. & Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm fucntion. Cell Motil. Cytoskeleton 8, 68–75 (1987).
Takahashi, K. & Kamimura, S. Dynamic aspects of microtubule sliding in sperm flagella. J. Submicrosc. Cytol. 15, 1–3 (1983).
Oiwa, K. & Takahashi, K. The force-velocity relationship for microtubule sliding in demembranated sperm flagella of the sea urchin. Cell Struct. Funct. 13, 193–205 (1988).
Kamimura, S. & Kamiya, R. High-frequency nanometre-scale vibration in ‘quiescent’ flagellar axonemes. Nature 340, 476–478 (1989).
Shingyoji, C. et al. Dynein arms are oscillating force generators. Nature 393, 711–714 (1998).
Block, S. M., Goldstein, L. S. B. & Schnapp, B. J. Bead movement by single kinesin moelcules studied with optical tweezers. Nature 348, 348–352 (1990).
Uyeda, T. Q. P., Warrick, H. M., Kron, S. J. & Spudich, J. A. Quantized velocities at low myosin densities in an in vitro motility assay. Nature 352, 307–311 (1991).
Goodenough, U. W. et al. High-pressure liquid chromatography fractionation of Chlamydomonas dynein extracts and characterization of inner-arm dynein subunits. J. Mol. Biol. 194, 481–494 (1987).
Goodenough, U. & Heuser, J. Structural comparison of purified dynein proteins with in situ dynein arms. J. Mol. Biol. 180, 1083–1118 (1984).
Amos, L. A. Brain dynein crossbridges microtubules into bundles. J. Cell Sci. 93, 19–28 (1989).
Samso, M., Radermacher, M., Frank, J. & Koonce, M. P. Structural characterization of a dynein motor domain. J. Mol. Biol. 276, 927–937 (1998).
Kagami, O. & Kamiya, R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonas inner-arm dynein. J. Cell Sci. 103, 653–664 (1992).
Gee, M. A., Heuser, J. E. & Vallee, R. B. An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636–639 (1997).
Funatsu, T. et al. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559 (1995).
Oiwa, K. et al. Microscopic kinetic measurements of single Cy3-EDA-ADP molecules interacting with myosin filaments in vitro. Biophys. J. 72, A180 (1997).
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Hancock, W. O. & Howard, J. Processivity of the motor protein kinesin requires two heads. J. Cell Biol. 140, 1395–1405 (1998).
Howard, J. & Hyman, A. A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence microscopy. Methods Cell Biol. 39, 105–113 (1993).
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Paschal, B. M., Obar, R. A. & Vallee, R. B. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature 342, 569–572 (1989).
King, S. M., Otter, T. & Witman, G. B. Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 134, 291–306 (1986).
Vallee, R. B. Reversible assembly purification of microtubules without assembly-promoting agents and further purification of tubulin, microtubule-associated proteins, and MAP fragments. Methods Enzymol. 134, 89–104 (1986).
Katayama, E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J. Biochem. 106, 751–770 (1989).
Jameson, D. M. & Eccleston, J. F. Fluorescent nucleotide analogs: Synthesis and applications. Methods Enzymol. 278, 363–390 (1997).
Acknowledgements
We thank M. Anson for discussion and comments on the manuscript and M.Kikumoto for single molecule measurements. This work was partly supported by the Hyogo Science and Technology Foundation (K.O. and H.K.) and a grant-in-aid from the Ministry of Education, Science and Culture of Japan (K.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakakibara, H., Kojima, H., Sakai, Y. et al. Inner-arm dynein c of Chlamydomonas flagella is a single-headed processive motor. Nature 400, 586–590 (1999). https://doi.org/10.1038/23066
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23066
This article is cited by
-
Versatile properties of dynein molecules underlying regulation in flagellar oscillation
Scientific Reports (2023)
-
Overview of the mechanism of cytoskeletal motors based on structure
Biophysical Reviews (2018)
-
Structural mechanism of the dynein power stroke
Nature Cell Biology (2014)
-
Non-Processive Force Generation by Mammalian Axonemal Dynein In Situ on Doublet Microtubules
Cellular and Molecular Bioengineering (2013)
-
Large-scale vortex lattice emerging from collectively moving microtubules
Nature (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.