Abstract
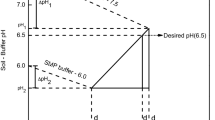
THE purpose of this note is to call attention to the fact that usual methods of measuring soil pH1 seriously underestimate the pH of very acid soils, such as those of coal mine spoils2, cat clays3 and solfataras4, in which acidity is due to the presence of free sulphuric acid. Standard methods for measuring soil pH involve making a slurry of soil with (usually) distilled water at a 1 : 2, 1 : 5, or 1 : 10 dilution, and reading the pH of this slurry. Implicit in this procedure is the assumption that the soil is buffered and hence that pH does not change with dilution. This assumption is erroneous in the case of the sulphuric acid soils.
Similar content being viewed by others
Article PDF
References
Peech, M., in Methods of Soil Analysis, Part 2, 914 (Amer. Soc. Agronomy Publ. No. 9, Madison, Wisconsin, 1965).
Kohnke, H., Adv. Agronomy, 2, 318 (1950).
Moorman, F. R., Soil Sci., 95, 271 (1963).
Schoen, R., Geol. Soc. Amer. Bull., 80, 643 (1969).
Doemel, W. N., and Brock, T. D., Arch. Mikrobiol., 72, 326 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DOEMEL, W., BROCK, T. pH of Very Acid Soils. Nature 229, 574 (1971). https://doi.org/10.1038/229574a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/229574a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.