Abstract
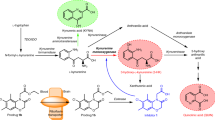
IT is generally assumed that the pharmacological actions of L-dopa (L-3,4-dihydroxyphenylalanine) are mediated through its conversion to dopamine. Thus, the specific value of L-dopa in the treatment of Parkinson's disease1–4 would lie in its ability to traverse the blood–brain barrier and undergo de-carboxylation in the parenchymal cells of the brain. It is likely that this mechanism operates physiologically in certain systems. But is dopamine the only metabolite mediating the action of L-dopa4–7? Some other simple derivative, formed in the brain by “conventional” pathways, may be pharmacologically active and responsible for the stimulation of dopamine-sensitive receptors that have been deprived of their normal presynaptic connexions through degenerative processes, as in Parkinson's disease, or by stereotaxically placed lesions in experimental animals8–10. Another possibility lies in the conversion of L-dopa or one of its immediate products to some quite different compound, for example by condensation of dopamine with an aldehyde to form a tetrahydroisoquinoline. Reactions of this type are known for pyridoxal and its phosphate11–13, as well as for 3,4-dihydroxyphenylacetaldehyde. The last compound arises from dopamine through the action of monoamine oxidase, and can condense non-enzymatically with dopamine to form tetrahydropapaveroline (nor-laudano-soline)14 as shown in Fig. 1. A Scruff's base is an intermediary in the reaction. Tetrahydropapaveroline possesses hypotensive activity14. It is conceivable that a benzyltetrahydroisoquinoline, derived from L-dopa in vivo, has at least some of the pharmacological activities ascribed to this amino-acid in Parkinson's disease7. The type reaction described here has been demonstrated in vivo;, in this case, 1-methyl-l,2,3,4-tetrahydroisoquinolines are formed15,16 by the condensation of catecholamines with acetaldehyde, the immediate dehydrogenation product of ethanol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Birkmayer, W., and Hornykiewicz, O., Arch. Psychiat. Nerv. Krankh., 203, 560 (1962).
Barbeau, A., Murphy, G. F., and Sourkes, T. L., in Monoamines et Système Nerveux Central, 247 (edit. by de Ajuriaguerra, J.) (Masson, Paris, 1962).
Cotzias, G. C., Van Woert, M. H., and Schiffer, L. M., New Engl. J. Med., 276, 374 (1967).
Calne, D. B., and Sandler, M., Nature, 226, 21 (1970).
Sourkes, T. L., Proc. Symp. Biochem. Chemother. Parkinson's Disease, 157th National Meeting Amer. Chem. Soc. April 1969, Minneapolis, Minnesota.
Sourkes, T. L., Biochem. Med., 3, 321 (1970).
Sourkes, T. L., Proc. Laurentian Meeting on Dopa in Parkinsonism (in the press).
Poirier, L. J., and Sourkes, T. L., J. Physiol. (Paris), 56, 426 (1964).
Poirier, L. J., and Sourkes, T. L., Brain, 88, 192 (1965).
Sourkes, T. L., and Poirier, L. J., Canad. Med. Assoc. J., 94, 53 (1966).
Schott, H. F., and Clark, W. G., J. Biol. Chem., 196, 449 (1952).
Sourkes, T. L., Arch. Biochem. Biophys., 51, 444 (1954).
Sourkes, T. L., Rev. Canad. Biol., 14, 49 (1955).
Holtz, P., Stock, K., and Westermann, E., Nature, 203, 656 (1964).
Davis, V. E., and Walsh, M. J., Science, 167, 1005 (1970).
Cohen, G., and Collins, M., Science, 167, 1749 (1970).
Shamma, M., in The Alkaloids (edit. by Manske, R. H. F.), 9, 1 (Academic Press, London, 1967).
Barton, D. H. R., and Cohen, T., in Festschrift Prof. Dr. Arthur Stoll, 117 (Birkhäuser, Basle, 1957).
Peng, M. T., J. Pharmacol. Exp. Ther., 139, 345 (1963).
Ernst, A. M., and Smelik, P. G., Experientia, 22, 837 (1966).
Anden, N. E., Rubensson, A., Fuxe, K., and Hökfelt, T., J. Pharm. Pharmacol., 19, 627 (1967).
Roos, B. E., J. Pharm. Pharmacol., 21, 263 (1969).
Ungerstedt, U., Butcher, L. L., Butcher, S. G., Anden, N. E., and Fuxe, K., Brain Res., 14, 461 (1969).
Schwab, R. S., Amador, L. V., and Letvin, J. Y., Trans. Amer. Neurol. Assoc., 76, 251 (1951).
Cotzias, G. C., Papavasiliou, P. S., Fehling, C., Kaufman, B., and Mena, I., New Engl. J. Med., 282, 31 (1970).
De Jong, H. H., Experimental Catalonia (Williams and Wilkins Co. Baltimore, 1945).
Divry, P., and Evrard, E., J. Belg. Neurol. Psychiat., 34, 506 (1934).
Ernst, A. M., Psychopharmacologia, 7, 391 (1965).
Nagatsu, T., Levitt, M., and Udenfriend, S., J. Biol Chem., 239, 2910 (1964).
Bédard, P., Larochelle, L., Poirier, L. J., and Sourkes, T. L., Canad. J. Physiol. Pharmacol., 48, 82 (1970).
Ingram, W. R., and Ranson, S. W., Arch. Neurol. Psychiat., 31, 987 (1934).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SOURKES, T. Possible New Metabolites mediating Actions of L-Dopa. Nature 229, 413–414 (1971). https://doi.org/10.1038/229413a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/229413a0
This article is cited by
-
1-Methyl-1,2,3,4-Tetrahydroisoquinoline, an Endogenous Amine with Unexpected Mechanism of Action: New Vistas of Therapeutic Application
Neurotoxicity Research (2014)
-
Biosynthesis of salsolinol, a tetrahydroisoquinoline alkaloid, in healthy subjects
Journal of Neural Transmission (1990)
-
Dopamine-derived alkaloids in alcoholism and in Parkinson's and Huntington's diseases
Journal of Neural Transmission (1988)
-
Chronic L-DOPA treatment of mice: A behavioural and biochemical study
Journal of Neural Transmission (1981)
-
Subacute l-DOPA in mice: Biochemical and behavioural effects
Psychopharmacology (1980)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.