Abstract
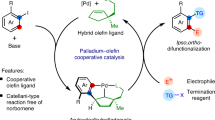
THERE is an interesting difference between the stereochemistry of nucleophilic attack on chelating diolefins and mono-olefins coordinated to platinum(II) or palladium(II), because the former give trans-addition of nucleophiles across the double bond (reaction (1))1–4, whereas the kinetic results for the oxidative hydrolysis of mono-olefins coordinated to palladium(II) are only consistent with cis-addition5,6. Although at 25° C di-olefin complexes of palladium(II) give trans-addition of methoxide ion to the double-bond and no reduction to the metal7, at higher temperatures reduction to palladium metal is observed8 and vinyl ethers—the normal oxidative-addition products9—are isolated7. Thus for di-olefin complexes, trans-addition is favoured at low temperatures, but for mono-olefin complexes cis-addition is favoured. In the oxidative acetylation of mono-olefins coordinated to palladium(II) both cis and trans-addition mechanisms occur simultaneously10.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stille, J. K., Morgan, R. A., Whitehurst, D. D. and Doyle, J. R., J. Amer. Chem. Soc., 87, 3282 (1965).
Stille, J. K., and Morgan, R. A., J. Amer. Chem. Soc., 88, 5135 (1966).
Green, M., and Hancock, R. I., J. Chem. Soc. (A), 2054 (1967).
Anderson, C. B., and Burreson, B. J., J. Organometal. Chem., 7, 181 (1967).
Henry, P. M., J. Amer. Chem. Soc., 86, 3246 (1964).
Henry, P. M., J. Amer. Chem. Soc., 88, 1595 (1966).
Schultz, R. G., J. Organometal. Chem., 6, 435 (1966).
Chatt, J., Vallarino, L. M., and Venanzi, L. M., J. Chem. Soc., 3413 (1957).
Stern, E. W., and Spector, M. L., Proc Chem. Soc., 370 (1961).
Schultz, R. G., and Gross, D. E., Adv. Chem., 70, 97 (1968).
Shaw, B. L., Chem. Commun., 464 (1968).
Denning, R. G., Hartley, F. R., and Venanzi, L. M., J. Chem. Soc. (A), 324 (1967).
Smidt, J., Chem. Ind., 54 (1962).
Holloway, C. E., Hulley, G., Johnson, B. F. G., and Lewis, J., J. Chem. Soc. (A), 53 (1969).
Lokken, S. J., and Martin, D. S., Inorg. Chem., 2, 562 (1963).
Maricic, S., Redpath, C. R., and Smith, J. A. S., J. Chem. Soc., 4905 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HARTLEY, F. Stereochemistry of Nucleophilic Attack on Olefins coordinated to Platinum(II) and Palladium(II). Nature 223, 615–616 (1969). https://doi.org/10.1038/223615a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/223615a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.