Abstract
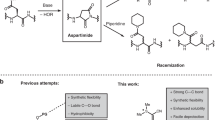
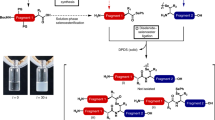
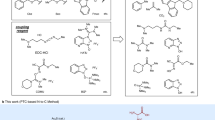
THE simplified procedure for peptide synthesis in which a basic “handle”, incorporated into the C-terminal residue, facilitates the extraction of the growing peptide after each coupling step1–3 has now been examined in a synthesis of Val5–angiotensin II. The basic “handle” was provided by the 4-picolyl ester of the C-terminal phenylalanine; this group can conveniently be removed by catalytic hydrogenation or by electrolytic reduction (unpublished work of D. Stevenson and W. B. Watkins). An analogous method using p-dimethylarninoazobenzyl esters has recently been reported4. The synthetic scheme is shown in Fig. 1. Coupling reactions (using 1.05–3.0 M proportions of acylating agent) were continued until no unchanged amino-component could be detected by thin-layer chromatography (limit of detection, 0.1 per cent); the histidine and arginine residues were incorporated by means of dicyclohexylcarbodiimide. The basic coupling product was extracted into 2 N-citric acid solution, except in the final stage, when the product was taken up on sulpho-ethyl-‘Sephadex’ saturated with 3-bromopyridine2. So effective were these simple separations that at every step the crude protected peptide was chromatographically pure (in at least four solvents) and had satisfactory elemental analysis. The overall yield of protected octapeptide (based on L-phenylalanine 4-picolyl ester dihydrobromide) was 34 per cent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Camble, R., Garner, R., and Young, G. T., Nature, 217, 247 (1968).
Garner, R., Schafer, D. J., Watkins, W. B., and Young, G. T., Peptides, Proc. Ninth Europ. Peptide Symp., Orsay (edit. by Bricas, E.), 145 (North-Holland, Amsterdam, 1968).
UK Patent Applications 40286/67, 17321/68, assigned to the National Research Development Corporation.
Wieland, Th., and Racky, W., Chimia, 22, 375 (1968).
Schwyzer, R., Iselin, B., Kappeler, H., Riniker, B., Rittel, W., and Zuber, H., Helv. Chim. Acta, 41, 1287 (1958).
Arakawa, K., and Bumpus, F. M., J. Amer. Chem. Soc., 83, 728 (1961); Marshall, G. R., and Merrifield, R. B., Biochemistry, 4, 2394 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GARNER, R., YOUNG, G. Facilitation of Peptide Synthesis by the Use of 4-Picolyl Esters: Synthesis of Val5–Angiotensin II. Nature 222, 177–178 (1969). https://doi.org/10.1038/222177a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/222177a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.