Abstract
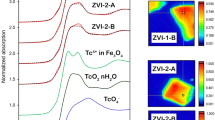
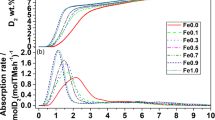
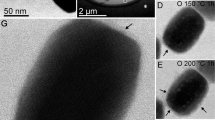
X-ray diffraction and kinetic measurements show that the rate of oxidation of magnetite crystals smaller than about 0.2µ to γ-Fe2O3 is controlled by the diffusion of iron cations. A model of the mechanism is successful in describing the kinetics of the process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hägg, G., Z. Physical. Chem., B29, 95 (1935).
Feitknecht, W., and Lehmann, H. W., Helv. Chim. Acta, 42, 2035 (1959).
Mackay, A. L., in Reactivity of Solids (edit. by de Boer, J. H.) (Elsevier, Amsterdam, 1960).
Egger, K., and Feitknecht, W., Helv. Chim. Acta, 45, 2042 (1962).
Feitknecht, W., and Mannweiler, U., Helv. Chim. Acta, 50, 570 (1967).
Feitknecht, W., Pure and Applied Chem., 9, 423 (1964).
Mannweiler, U., Chimica, 20, 363 (1966).
van Oosterhout, G. W., and Rooymans, C. J. M., Nature, 181, 44 (1948).
Gallagher, K. J., and Phillips, D. N., Trans. Faraday Soc., 64, 785 (1968).
Hyde, B. G., Bevan, D. J. M., and Eyring, L., Phil. Trans. Roy. Soc., A, 259, 583 (1966).
Hoekstra, H. R., Santoro, A., and Siegel, S., J. Inorg. Nucl. Chem., 18, 166 (1961).
Colombo, U., Science, 147, 1033 (1965); Nature, 202, 175 (1964).
DeBoer, F. E., and Selwood, P. W., J. Amer. Chem. Soc., 76, 3365 (1954).
Kochendörfer, A., Z. Krist., 105, 393 (1944).
McKay, A. T., Proc. Phys. Soc., 42, 547 (1930).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GALLAGHER, K., FEITKNECHT, W. & MANNWEILER, U. Mechanism of Oxidation of Magnetite to γ-Fe2O3. Nature 217, 1118–1121 (1968). https://doi.org/10.1038/2171118a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2171118a0
This article is cited by
-
O2 adsorption on Fe3O4 (110) surface and effect of gangue element Al doping: combined study of binding experiment and ab initio molecular dynamics
Journal of Iron and Steel Research International (2024)
-
Photocatalytic activity of Fe3O4–Fe2O3 particles supported on mordenite under visible light exposure for methylene blue degradation
SN Applied Sciences (2023)
-
A simple method to form a forest of carbon nanotube bundles during growth stage
SN Applied Sciences (2022)
-
Insight on thermal stability of magnetite magnetosomes: implications for the fossil record and biotechnology
Scientific Reports (2020)
-
Oxidation induced strain and defects in magnetite crystals
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.