Abstract
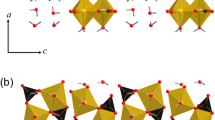
THE orthorhombic anthophyllite minerals and the monoclinic cummingtonites and grunerites are members of two isomorphous amphibole series of composition (Mg,Fe2+)7Si8O22(OH)2. (All anthophyllites considered here have less than one aluminium (III) ion per formula unit3.) Anthophyllites have been described1,2 with 0–3.28 iron (II)* ions per formula unit, while compositions between 2.02 and 6.67 iron (II) ions have been recorded in the cummingtonite–grunerite series2,3, which implies the existence of some compositional overlap between the two series. Aluminium-rich anthophyllites (gedrites) show a much wider range of iron concentration, ranging up to Fe5Al2-(Al2Si6)O22(OH)2 (ref. 2).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rabbitt, J. C., Amer. Min., 33, 263 (1948).
Deer, W. A., Howie, R. A., and Zussman, J., Rock-Forming Minerals, 2, 216, 236, 212 (Longmans, London, 1963).
Mueller, R. F., Amer. J. Sci., 258, 449 (1960).
Warren, B. E., and Modell, D. I., Z. Krist., 75, 161 (1930).
Ghose, S., Acta Cryst., 14, 622 (1961).
Fischer, K. F., Amer. Min., 49, 963 (1966).
Whittaker, E. J. W., Acta Cryst., 13, 291 (1960).
Ghose, S., Amer. Min., 47, 388 (1962); Min. Mag., 35, 46 (1965).
Bancroft, G. M., Burns, R. G., and Maddock, A. G., Amer. Min. (in the press).
Burns, R. G., and Strens, R. G. J., Science, 153, 890 (1966). Strens, R. G. J., Chem. Commun., 519 (1966).
Bancroft, G. M., Maddock, A. G., and Ward, J., Chem. and Indust., 423 (1966).
Rabbitt, J. C., Amer. Min., 33, 263 (1948).
Tilley, C. E., Amer. Min., 42, 412 (1957).
Stone, A. J., appendix to Bancroft, G. M., Maddock, A. G., Ong, W. K., and Prince, R. H. (in preparation).
Bancroft, G. M., Burns, R. G., and Maddock, A. G., Geochim. et Cosmochim. Acta (in preparation).
Boyd, F. R., in Researches in Geochemistry (edit. by Abelson, P. H.), 391 (J. Wiley and Sons, New York, 1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BANCROFT, G., MADDOCK, A., BURNS, R. et al. Cation Distribution in Anthophyllite from Mössbauer and Infra-red Spectroscopy. Nature 212, 913–915 (1966). https://doi.org/10.1038/212913a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/212913a0
This article is cited by
-
Phlogopite-pargasite coexistence in an oxygen reduced spinel-peridotite ambient
Scientific Reports (2021)
-
Mineral Mössbauer spectroscopy: Correlations between chemical shift and quadrupole splitting parameters
Hyperfine Interactions (1994)
-
Mössbauer spectrometry as a tool for mining industry: Application to an uranium-bearing mineralization of albitized granite
Hyperfine Interactions (1989)
-
Compositional dependence of the hyperfine interaction of 57Fe in anthophyllite
Physics and Chemistry of Minerals (1977)
-
Synthesis, lattice constants and OH-valence vibrations of an orthorhombic amphibole with excess OH in the system Li2O-MgO-SiO2-H2O
Contributions to Mineralogy and Petrology (1976)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.