Abstract
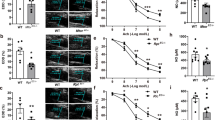
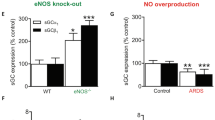
Endothelial nitric oxide synthase (eNOS) is the nitric oxide synthase isoform responsible for maintaining systemic blood pressure, vascular remodelling and angiogenesis1,2,3,4. eNOS is phosphorylated in response to various forms of cellular stimulation5,6,7 but the role of phosphorylation in the regulation of nitric oxide (NO) production and the kinase(s) responsible are not known. Here we show that the serine/threonine protein kinase Akt (protein kinase B) can directly phosphorylate eNOS on serine 1179 and activate the enzyme, leading to NO production, whereas mutant eNOS (S1179A) is resistant to phosphorylation and activation by Akt. Moreover, using adenovirus-mediated gene transfer, activated Akt increases basal NO release from endothelial cells, and activation-deficient Akt attenuates NO production stimulated by vascular endothelial growth factor. Thus, eNOS is a newly described Akt substrate linking signal transduction by Akt to the release of the gaseous second messenger NO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shesely, E. G. et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 93, 13176–13181 (1996).
Huang, P. L. et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995).
Rudic, R. D. et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 101, 731–736 (1998).
Murohara, T. et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Invest. 101, 2567–2578 (1998).
Michel, T., Li, G. K. & Busconi, L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 90, 6252–6256 (1993).
Garcia-Cardena, G., Fan, R., Stern, D. F., Liu, J. & Sessa, W. C. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. 271, 27237–27240 (1996).
Corson, M. A. et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 79, 984–991 (1996).
Coffer, P. J., Jin, J. & Woodgett, J. R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335, 1–13 (1998).
Franke, T. F., Kaplan, D. R. & Cantley, L. C. PI3K: downstream AKTion blocks apoptosis. Cell 88, 435–437 (1997).
Downward, J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10, 262–267 (1998).
Murga, C., Laguinge, L., Wetzker, R., Cuadrado, A. & Gutkind, J. S. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase gamma. J. Biol. Chem. 273, 19080–19085 (1998).
Dimmeler, S., Assmus, B., Hermann, C., Haendeler, J. & Zeiher, A. M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 83, 334–341 (1998).
Cardone, M. H. et al. Regulation of cell death protease caspase-9 by phosphorylation. Science 282, 1318–1321 (1998).
Zeng, G. & Quon, M. J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Invest. 98, 894–898 (1996).
Papapetropoulos, A., Garcia-Cardena, G., Madri, J. A. & Sessa, W. C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 100, 3131–3139 (1997).
Parenti, A. et al. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J. Biol. Chem. 273, 4220–4226 (1998).
Liu, J., Hughes, T. E. & Sessa, W. C. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the golgi region of cells: A green fluorescent protein study. J. Cell Biol. 137, 1525–1535 (1997).
García-Cardeña, G., Oh, P., Liu, J., Schnitzer, J. E. & Sessa, W. C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc. Natl Acad. Sci. USA 93, 6448–6453 (1996).
Shaul, P. W. et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 271, 6518–6522 (1996).
Sessa, W. C. et al. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J. Biol. Chem. 270, 17641–17644 (1995).
Liu, J., Garcia-Cardena, G. & Sessa, W. C. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry 35, 13277–13281 (1996).
Kantor, D. B. et al. Arole for endothelial NO synthase in LTP revealed by adenovirus- mediated inhibition and rescue. Science 274, 1744–1748 (1996).
Gerber, H. P. et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Requirement for flk-1/kdr activation. J. Biol. Chem. 273, 30336–30343 (1998).
Kuchan, M. J. & Frangos, J. A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 266, C628–636 (1994).
Ayajiki, K., Kindermann, M., Hecker, M., Fleming, I. & Busse, R. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ. Res. 78, 750–758 (1996).
Garcia-Cardena, G. et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392, 821–824 (1998).
Yano, S., Tokumitsu, H. & Soderling, T. R. Calcium promotes cell survival through CaM-K kinase activation of the protein kinase-B pathway. Nature 396, 584–587 (1998).
Salerno, J. C. et al. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. J. Biol. Chem. 272, 29769–29777 (1997).
Smith, R. C. et al. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 11, 1674–1689 (1997).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 (1996).
Acknowledgements
We thank P. Martasek and B. S. Masters for recombinant eNOS; J. Liu for constructing and characterizing the myr-nNOS construct; D. Bredt and T. Billiar for NOS cDNAs; T.Zioncheck for human VEGF; Y. Chen for generating the eNOS S1179A E. Coli expression construct; and K. Williams, M. LoPresti and K. Stone in the Keck Facility for their help with identification of the eNOS phosphopeptides. This work was supported by grants from the NIH and American Heart Association. W.C.S. is an Established Investigator of the American Heart Association. J.P.G. is supported by fellowships from the Heart and Stroke Foundation of Canada and from FCAR.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Fulton, D., Gratton, JP., McCabe, T. et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 (1999). https://doi.org/10.1038/21218
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/21218
This article is cited by
-
Estrogen-receptor status determines differential regulation of α1- and α2-adrenoceptor-mediated cell survival, angiogenesis, and intracellular signaling responses in breast cancer cell lines
Medical Oncology (2024)
-
VEGF-A-induced changes in distal outflow tract structure and function
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Capillary leak and endothelial permeability in critically ill patients: a current overview
Intensive Care Medicine Experimental (2023)
-
Ginkgolide B caused the activation of the Akt/eNOS pathway through the antioxidant effect of SOD1 in the diabetic aorta
Pflügers Archiv - European Journal of Physiology (2023)
-
Effect of 3-caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea on endothelial nitric oxide synthase activation via calcium signaling pathway
Toxicological Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.