Abstract
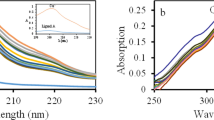
DURING an examination of hydrocarbon electrodes, evidence was obtained which confirms that analytical reagent grade phosphoric and sulphuric acids contain an oxidizable impurity. The cell and procedures used will be described elsewhere1. In Fig. 1 is shown a plot of potential versus log apparent current density for platinized platinum immersed in nitrogen-stirred unpre-electrolysed 5MH3PO4 at 80° C (inverted triangles). Prior to these measurements the system had not been in contact with a hydrocarbon or any other oxidizable material. The slope of the linear part of the curve is 143 mV. After an anodic pre-electrolysis (60 h at an anodic current density of 62 m.amp/cm2), the Tafel behaviour was no longer observed with nitrogen stirring, and a limiting current was reached (triangles, Fig. 1). When the purified acid was stirred with ebhylene at 80° C, the polarization curve obtained (circles, Fig. 1) was identical with that obtained with nitrogen stirring of the unpre-electrolysed acid. Similar data obtained with 3N H2SO4 are shown in Fig. 2. The slope of the linear parts of the curves in this case is 183 mV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Thacker, R. (to be published).
Wroblowa, H., Piersma, B. J., and Bockris, J. O'M, J. Electroanal. Chem., 6, 401 (1963).
Bockris, J. O'M, Piersma, B. J., and Gileadi, E., paper presented at Electrochemical Society Meeting, October 1964, Extended Abst. No. 12.
Green, M., Weber, J., and Drazic, V., J. Electrochem. Soc., 111, 721 (1964).
Johnson, J. W., Wroblowa, H., and Bockris, J. O'M, J. Electrochem. Soc., 111, 863 (1964).
Oxley, J. E., Johnson, G. K., and Buzalski, B. T., Electrochimica Acta, 9, 897 (1964).
Hoare, J. P., J. Electrochem. Soc., 109, 858 (1962).
Hoare, J. P. (personal communication).
Schuldiner, S., and Roe, R. M., J. Electrochem. Soc., 110, 1142 (1963).
Damjanovic, A., Rao, M. L. B., and Genshaw, M., ASTIA Rep. No. AD 405675 (November 1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
THACKER, R. Use of Phosphoric and Sulphuric Acids in Hydrocarbon Electrode Investigations. Nature 207, 856–857 (1965). https://doi.org/10.1038/207856a0
Issue Date:
DOI: https://doi.org/10.1038/207856a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.