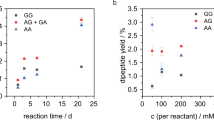
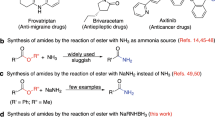
Abstract
THE reduction of esters by sodium in liquid ammonia solutions has been investigated in some detail by Kharasch et al.1. From an analysis of the products obtained, it was concluded by these authors that aliphatic esters were converted to a mixture of the corresponding alcohol, amide and acid, in addition to varying amounts of unidentified substances. There have been very few investigations of the reaction of amino-acid esters with sodium in liquid ammonia solutions. McChesney and Miller2 treated glycine ethyl ester hydrochloride with sodium in liquid ammonia and obtained some of the glycine ester and an unidentified product. Benzyl esters of amino-acids and peptides are known3 to be cleaved to form the corresponding amino-acid or peptide. With the availability of the t-butyloxycarbonyl group for masking ammo functions and the t-butyl group for protecting carboxyl groups and hydroxyl groups, it became important to know whether these groups were resistant to the reductive action of sodium in liquid ammonia. This treatment readily removes other protecting groups such as the p-toluene sulphonyl and the carbobenzoxy groups. Anderson and McGregor4 found that the t-butyloxy-carbonyl group is resistant to treatment with sodium in liquid ammonia. The effects of this reductive treatment on amino-acid and peptide t-butyl esters have been investigated. Results of the reaction of proline t-butyl ester with sodium in liquid ammonia are presented here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kharasch, M. S., Sternfeld, E., and Mayo, F. R., J. Org. Chem., 5, 362 (1940).
McChesney, E. W., and Miller, C. O., J. Amer. Chem. Soc., 53, 3888 (1931).
Roberts, C. W., J. Amer. Chem. Soc., 76, 6203 (1954).
Anderson, G. W., and McGregor, A. C., J. Amer. Chem. Soc., 79, 6180 (1957).
Anderson, G. W., and Callahan, F. M., J. Amer. Chem. Soc., 82, 3359 (1960).
Spackman, D. H., Stein, W. H., and Moore, S., Anal. Chem, 30, 1190 (1958).
Zahn, H., and Rexroth, E., Z. Anal. Chem., 148, 181 (1955).
Li, C. H., Ramachandran, J., and Chung, D., J. Amer. Chem. Soc., 86, 2711 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RAMACHANDRAN, J. Reaction of Amino-acid Esters with Sodium in Liquid Ammonia : Cleavage of the Proline Ring. Nature 206, 927–928 (1965). https://doi.org/10.1038/206927a0
Issue Date:
DOI: https://doi.org/10.1038/206927a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.