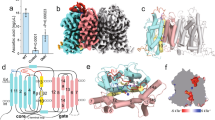
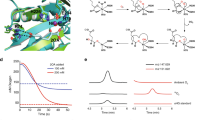
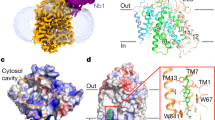
Abstract
Vitamin C (L-ascorbic acid) is essential for many enzymatic reactions, in which it serves to maintain prosthetic metal ions in their reduced forms (for example, Fe2+, Cu+)1,2, and for scavenging free radicals in order to protect tissues from oxidative damage3. The facilitative sugar transporters of the GLUT type can transport the oxidized form of the vitamin, dehydroascorbic acid4,5,6, but these transporters are unlikely to allow significant physiological amounts of vitamin C to be taken up in the presence of normal glucose concentrations, because the vitamin is present in plasma essentially only in its reduced form7. Here we describe the isolation of two L-ascorbic acid transporters, SVCT1 and SVCT2, from rat complementary DNA libraries, as the first step in investigating the importance of L-ascorbic acid transport in regulating the supply and metabolism of vitamin C. We find that SVCT1 and SVCT2 each mediate concentrative, high-affinity L-ascorbic acid transport that is stereospecific and is driven by the Na+ electrochemical gradient. Despite their close sequence homology and similar functions, the two isoforms of the transporter are discretely distributed: SVCT1 is mainly confined to epithelial systems (intestine, kidney, liver), whereas SVCT2 serves a host of metabolically active cells and specialized tissues in the brain, eye and other organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Englard, S. & Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 6, 365–406 (1986).
Padh, H. Vitamin C: newer insights into its biochemical functions. Nutr. Rev. 49, 65–70 (1991).
Rose, R. C. & Bode, A. M. Biology of the free radical scavengers: an evaluation of ascorbate. FASEB J. 7, 1135–1142 (1993).
Vera, J. C., Rivas, C. I. & Fischbarg, J. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364, 79–82 (1993).
Rumsey, S. C. et al . Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 272, 18982–18989 (1997).
Agus, D. B. et al . Vitamin C crosses the blood-brain barrier in the oxidized from through the glucose transporters. J. Clin. Invest. 100, 2842–2848 (1997).
Dhariwal, K. R., Hartzell, W. O. & Levine, M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 54, 712–716 (1991).
Nagase, T. et al . Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201 -KIAA0280 ) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 3, 321–354 (1996).
Guimaraes, M. J. et al . Anew approach to the study of haematopoietic development in the yolk sac and embryoid bodies. Development 121, 3335–3346 (1995).
Diallinas, G., Gorfinkiel, L., Arst, H. N., Cecchetto, G. & Scazzocchio, C. Genetic and molecular characterization of a gene encoding a wide specificity purine permease of Aspergillus nidulans reveals a novel family of transporters conserved in prokaryotes and eukaryotes. J. Biol. Chem. 270, 8610–8622 (1995).
Wright, E. M., Loo, D. D. F., Turk, E. & Hirayama, B. A. Sodium cotransporters. Curr. Opin. Cell Biol. 8, 468–473 (1996).
Hazama, A., Loo, D. D. F. & Wright, E. M. Presteady-state currents of the rabbit Na+/glucose cotransporter (SGLT1). J. Membr. Biol. 155, 175–186 (1997).
Mackenzie, B., Loo, D. D. F., Panayotova-Heiermann, M. & Wright, E. M. Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. J. Biol. Chem. 271, 32678–32683 (1996).
Mackenzie, B., Loo, D. D. F. & Wright, E. M. Relationships between Na+/glucose cotransporter currents and fluxes. J. Membr. Biol. 162, 101–106 (1998).
Gunshin, H. et al . Cloning and characterization of a proton-coupled mammalian metal-ion transporter. Nature 388, 482–488 (1997).
Eskandari, S. et al . Thyroid Na+/I symporter: mechanism, stoichiometry, and specificity. J. Biol. Chem. 272, 27230–27238 (1997).
Segel, I. H. Biochemical Calculations2nd edn(Wiley, New York, (1976).
Bowers-Komro, D. M. & McCormick, D. B. Characterization of ascorbic acid uptake by isolated rat kidney cells. J. Nutr. 121, 57–64 (1991).
Toggenburger, G. et al . Na+-dependent, potential-sensitive L-ascorbate transport across brush border membrane vesicles from kidney cortex. Biochim. Biophys. Acta 646, 433–443 (1981).
Helbig, H. et al . Electrogenic Na+-ascorbate cotransport in cultured bovine pigmented ciliary epithelial cells. Am. J. Physiol. 256, C44–C49 (1989).
Rose, R. C. & Wilson, J. X. in Vitamin C in Health and Disease(eds Packer, L. & Fuchs, J.) 143–161 (Dekker, New York, (1997).
Franceschi, R. T., Wilson, J. X. & Dixon, S. J. Requirement for Na+-dependent ascorbic acid transport in osteoblast function. Am. J. Physiol. 268, C1430–C1439 (1995).
Hammarström, L. Autoradiographic studies on the distribution of C14-labelled ascorbic acid and dehydroascorbic acid. Acta Physiol. Scand. 70 (suppl.)289, 1–75 (1966).
Rose, R. C. & Bode, A. M. Ocular ascorbate transport and metabolism. Comp. Biochem. Physiol. 100, 273–285 (1991).
Reiss, G. R., Werness, P. G., Zollman, P. E. & Brubaker, R. F. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch. Ophthalmol. 104, 753–755 (1986).
Kodama, T., Kabasawa, I., Tamura, O. & Reddy, V. N. Dynamics of ascorbate in the aqueous humor and tissues surrounding ocular chambers. Ophthalmic Res. 17, 331–337 (1985).
Romero, M. F., Kanai, Y., Gunshin, H. & Hediger, M. A. Expression cloning using Xenopus laevis oocytes. Methods Enzymol. 296, 17–52 (1998).
Mackenzie, B. in Biomembrane Transport(ed. Van Winkle, L. J.) 327–342 (Academic, San Diego, (1999).
Schaeren-Wiemers, N. & Gerfin-Moser, A. Asingle protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993).
Acknowledgements
We thank E. Brown for providing MC3T3-E1 cells and P. Fong for providing the pTLN2 vector. This research was supported by the NIH, the National Kidney Foundation, the American Heart Association Massachusetts Affiliate, Cooley's Anemia Foundation, and the International Human Frontier Science Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukaguchi, H., Tokui, T., Mackenzie, B. et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 399, 70–75 (1999). https://doi.org/10.1038/19986
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/19986
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.