Abstract
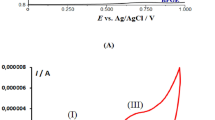
THE work of Gerischer1 and Delahay et al. 2 suggested to us the use of the hanging mercury-drop electrode as a rotating electrode. This electrode, used as a stationary electrode (as proposed by Ross et al. 3), offers several distinct advantages over the conventional dropping-mercury electrode. The charging current is largely reduced, the current-voltage curve can be scanned in a shorter time and the curve-distorting oscillations are eliminated. However, used as a rotating electrode its advantages are even greater. The electrode owes its greater sensitivity to the accelerated diffusion of reducible species (thinner diffusion layer). Peak-formation in the current-voltage curve is prevented, because depletion of the reducible species in the diffusion layer of the electrode cannot occur. If desired, the curve can be plotted manually: it resembles the polarogram in its ideal form. Analysis of multicomponent systems offers none of the extra difficulties encountered with the stationary type3, and compensation of the diffusion current of a more noble metal ion, preceding the diffusion current of the metal ion to be determined, is therefore possible. The reproducibility of repeated determinations on the same solution is comparable to or better than that of conventional polarography. It is possible to determine concentrations of reducible ions as low as 10−7 M.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gerischer, H., Z. physik. Chem., 202, 302 (1953).
Berzins, T., and Delahay, P., J. Amer. Chem. Soc., 77, 6448 (1955).
Ross, J. W., De Mars, R. D., and Shain, I., Anal. Chem., 28, 1768 (1956).
Mamantov, G., Papoff, P., and Delahay, P., J. Amer. Chem. Soc., 79, 4034 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BARENDRECHT, E. A Rotating Hanging Mercury-Drop Electrode. Nature 181, 764–765 (1958). https://doi.org/10.1038/181764a0
Issue Date:
DOI: https://doi.org/10.1038/181764a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.