Abstract
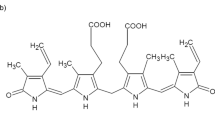
IT is generally accepted that all the naturally occurring ‘tetrapyrrolic’ bile pigments must be of a IX-α structure, that is, that they are derived from the biliverdin which is produced biologically by rupture of the α-methene bridge carbon atom of protoporphyrin IX. Therefore, all bile pigments prepared from biological material should possess a 1,3,6,7-tetramethyl-4,5-dicarboxyethyl-2,8-divinyl (or diethyl), open-chain, ‘tetrapyrrolic’ structure (using the system of numbering shown in formula (I). Although some of the bile pigments have been synthesized by methods which exclude the formation of isomers other than IX-α, there is, at present, no analytical evidence of the absence of such isomers in the pigments obtained from biological sources. The presence of such isomers would be expected if biological fission of the protoporphyrin molecule did not occur exclusively at the α-methene linkage. Since stercobilin (and therefore also the less-hydrogenated bile pigments) is known to be produced by at least two distinct physiological mechanisms1, such a possibility is not entirely speculative. The method of identification of pyrrole carboxylic acids described by one of us and used in investigations of the porphyrins2 has now been applied to a study of the order of the β-side-chains of the bile pigments. In the bile pigments one α-position of each end ring bears an oxygen atom (usually formulated in a hydroxyl group3) instead of a carbon atom. It is therefore impossible for these rings, on degradation, to provide the αα′-pyrroledicarboxylic acids given under similar conditions by the corresponding porphyrins; any such acids can only originate from the middle rings of the pigment molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gray, C. H., Neuberger, A., and Sneath, P. H. A., Biochem. J., 47, 87 (1950).
Nicolaus, R. A., Mangoni, L., and Caglioti, L., Annali di Chimica, 46, 793 (1956). Nicolaus, R. A., and Nicoletti, R., ibid., 47, 87 (1957). Nicolaus, R. A., Mangoni, L., and Nicoletti, R., ibid., 47, 178 (1957). Nicolaus, R. A., and Caglioti, L., Ricerca Scient., 27, 113 (1957). Nicolaus, R. A., and Nicoletti, R., ibid., 27, 113, 1527 (1957). Nicolaus, R. A., and Mangoni, L., ibid., 27, 1865 (1957).
Fischer, H., and Orth, H., “Die Chemie des Pyrrols” (1943).
Plieninger, H., and Decker, M., Liebigs Annalen, 598, 198 (1956).
Lemberg, R., and Legge, J. W., “Hæmatin Compounds and the Bile Pigments”, 103 (Interscience Publications, New York, 1949).
Gray, C. H., and Nicholson, D. C., Nature, 179, 264 (1957).
Gray, C. H., and Nicholson, D. C., Nature, 180, 336 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GRAY, C., NICHOLSON, D. & NICOLAUS, R. The IX-α Structure of the Common Bile Pigments. Nature 181, 183–185 (1958). https://doi.org/10.1038/181183b0
Issue Date:
DOI: https://doi.org/10.1038/181183b0
This article is cited by
-
Degradation of Haem Compounds to Bile Pigments
Nature New Biology (1972)
-
Gallenfarbstoffe bei wirbellosen Tieren
Die Naturwissenschaften (1970)
-
Isomeric Bile Pigments as Products of the In Vitro Fission of Hæmin
Nature (1962)
-
Dextrorotatory Urobilin-IXα and Racemic d-Urobilin
Nature (1958)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.