Abstract
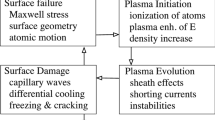
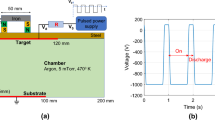
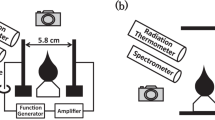
WHEN an arc discharge at very low pressure passes between metal electrodes the cathode experiences a force directed away from the anode. By suspending a copper cathode on a pendulum and observing the deflexion Tanberg1 measured this force. At the same time he measured the rate of evaporation of cathode material. With these data he obtained the velocity of the evaporating atoms from Newton's laws of motion. From a force of 20 dynes/amp. and a rate of evaporation of 10−5 gm./coulomb, he estimated that the copper atoms left the cathode with a velocity of about 106 cm./sec. He suggested from this that the cathode was at a temperature of about 5 × 105 deg. C. Up to the present his claim has caused some concern. Opinions are divided into two groups: those who question the accuracy of the experiments and those who disbelieve in Newton's laws. However, other evidence2–4 tends to support Tanberg's measurements.
Similar content being viewed by others
Article PDF
References
Tanberg, R., Phys. Rev., 35, 1080 (1930).
Robertson, R. M., Phys. Rev., 53, 578 (1938).
Kobel, E., Phys. Rev., 36, 1636 (1930).
Tonks, L., Phys. Rev., 50, 226 (1936).
Robson, A. E., and von Engel, A., Nature, 175, 646 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ROBSON, A., VON ENGEL, A. An Explanation of the Tanberg Effect. Nature 179, 625 (1957). https://doi.org/10.1038/179625a0
Issue Date:
DOI: https://doi.org/10.1038/179625a0
This article is cited by
-
The Tanberg Effect
Nature (1957)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.