Abstract
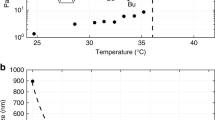
IT is well known that the stability factor of hydrophobic sols decreases, at first, exponentially with the ionic strength of the solution, and reaches, rather suddenly, its limiting value for a certain concentration C 1 of the coagulating electrolyte1. For concentrations lower than C 1 we have the so-called ‘slow coagulation’; the interaction energy consists of long-range electrical repulsions, dependent on ionic strength, and of Van der Waals attractions. For concentrations higher than C 1, the electrical repulsions have practically disappeared2 and we have ‘rapid coagulation’.
Similar content being viewed by others
Article PDF
References
Reerink, H., and Overbeek, J. T. G., Disc. Farad. Soc., 18, 74 (1954).
Verwey, E. J. W., and Overbeek, J. T. G., “Theory of the Stability of Lyophobic Colloids” (Elsevier, 1948).
Von Smoluchowski, M., Phys. Z., 17, 557, 585 (1916); Z. phys. Chem., 92, 129 (1918).
Hamaker, H. C., Physica, 4, 1058 (1937).
Kruyt, H. R., and Van Arkel, A. E., Rec. Trav. Chim., 39, 656 (1920); 40, 169 (1921).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DE BROUCKÈRE, L., WATILLON, A. & VAN GRUNDERBEECK, F. Existence of a Non-Electrostatic Stabilizing Factor in Hydrophobic Selenium Hydrosols. Nature 178, 589 (1956). https://doi.org/10.1038/178589a0
Issue Date:
DOI: https://doi.org/10.1038/178589a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.