Abstract
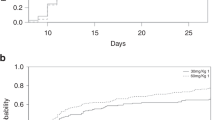
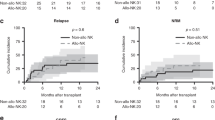
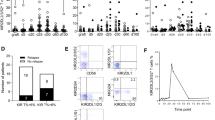
Although thymoglobulin and alemtuzumab are frequently used in hematopoietic stem cell transplantation (HSCT), little is known of their effects on NK cells, which mediate important functions in post-transplantation immunology. In the present study, we determined NK cell death in vitro using propidium iodide and Annexin V. The NK cell activity in 34 patients at day +30 after allogeneic HSCT was assessed using the CD107a assay. Alemtuzumab and thymoglobulin were similarly very potent in inducing NK cell death in vitro. Even in low concentrations (<1 μg/ml) the antibodies induced apoptosis and necrosis in a relevant percentage of NK cells (>30%). However, the number of tumor reactive (CD107a+) NK cells was 13.16 per μl and 1.15 per μl (mean) in patients receiving T-cell depletion with 6 mg/kg thymoglobulin and in patients receiving 100 mg alemtuzumab, respectively (P=0.02). Although thymoglobulin and alemtuzumab are equally NK cell toxic in vitro, the recovery of NK cell frequency and anti-tumor reactivity is reduced in recipients of alemtuzumab. Our findings can be explained by a longer half-life of alemtuzumab as compared to active thymoglobulin under therapeutic conditions. Prolonged immunosuppression with increased risk of infections and tumor relapse are a potential threat to patients undergoing HCST and receiving alemtuzumab as T-cell depletion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferrara JL, Deeg HJ . Graft-versus-host disease. N Engl J Med 1991; 324: 667–674.
Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol 2005; 129: 631–643.
Dodero A, Carrabba M, Milani R, Rizzo E, Raganato A, Montefusco V et al. Reduced-intensity conditioning containing low-dose alemtuzumab before allogeneic peripheral blood stem cell transplantation: graft-versus-host disease is decreased but T-cell reconstitution is delayed. Exp Hematol 2005; 33: 920–927.
Fehse N, Fehse B, Kroger N, Zabelina T, Freiberger P, Kruger W et al. Influence of anti-thymocyte globulin as part of the conditioning regimen on immune reconstitution following matched related bone marrow transplantation. J Hematother Stem Cell Res 2003; 12: 237–242.
Giebel S, Dziaczkowska J, Wojnar J, Krawczyk-Kulis M, Markiewicz M, Kruzel T et al. The impact of immunosuppressive therapy on an early quantitative NK cell reconstitution after allogeneic haematopoietic cell transplantation. Ann Transplant 2005; 10: 29–33.
Appelbaum FR . Haematopoietic cell transplantation as immunotherapy. Nature 2001; 411: 385–389.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Alter G, Malenfant JM, Altfeld M . CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22.
Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E et al. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 2005; 19: 835–840.
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH . Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84: 1415–1420.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C . A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39–51.
Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979; 54: 713–733.
Lozzio BB, Lozzio CB . Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk Res 1979; 3: 363–370.
Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003; 102: 814–819.
Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 2004; 104: 948–955.
Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G . Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy 2001; 3: 261–267.
Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant 2003; 9: 460–471.
Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis 2007; 44: 204–212.
Sandherr M, Einsele H, Hebart H, Kahl C, Kern W, Kiehl M et al. Antiviral prophylaxis in patients with haematological malignancies and solid tumours: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Oncology (DGHO). Ann Oncol 2006; 17: 1051–1059.
Charbonnier A, Sainty D, Faucher C, Arnoulet C, Chabannon C, Blaise D . Immune reconstitution after blood cell transplantation. Hematol Cell Ther 1997; 39: 261–264.
Lowdell MW, Craston R, Ray N, Koh M, Galatowicz G, Prentice HG . The effect of T cell depletion with Campath-1M on immune reconstitution after chemotherapy and allogeneic bone marrow transplant as treatment for leukaemia. Bone Marrow Transplant 1998; 21: 679–686.
Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H . Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood 1996; 88: 2775–2779.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penack, O., Fischer, L., Stroux, A. et al. Serotherapy with thymoglobulin and alemtuzumab differentially influences frequency and function of natural killer cells after allogeneic stem cell transplantation. Bone Marrow Transplant 41, 377–383 (2008). https://doi.org/10.1038/sj.bmt.1705911
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705911