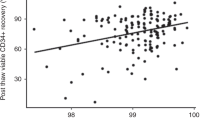
Abstract
Collection of PBSC by leukapheresis requires one venous access (VA) for inflow and one for outflow. The use of implantable venous access devices (IVAD) has never been reported in this setting. We retrospectively analyzed the use of IVAD for performing apheresis. The study was conducted between January 2000 and June 2005 on 64 patients (41 children) requiring intensification for treatment of a solid tumor. Mean body weight was 26 kg (range 8–91 kg) for a median age of 8.5 years (range 0.7–66 years). A total of 121 aphereses were performed (mean 1.89 apheresis/patient). The second VA was in a cubital vein in 84 procedures and was a temporary central VA in 31. Mean duration of apheresis was 3 h (range 30–274 min). Mean flow rate was 41.3 ml/min (range 12–85 ml/min). Mean collection rate was 59.2% for CD34+ cells and 70% for mononuclear cells. The total number of CD34+ cells collected was 2.5 × 106/kg per apheresis, and 5.9 × 106/kg per patient. Several complications occurred: one catheter-related sepsis (0.86%), four catheter occlusions (3.47%) and eight hemodynamic instabilities related to extracorporeal volume. Weight <10 kg is a risk factor for complication (P=0.0006). IVAD are effective and safe for PBSC collection. Placement of a second central VA (requiring general anesthesia for children) could be avoided.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gorlin JB, Humphreys D, Kent P, Galachi D, Kevy SV, Grupp S et al. Pediatric large volume peripheral blood progenitor cell collections from patients under 25 kg: a primer. J Clin Apher 1996; 11: 195–203.
Kevy SV, Fosburg M . Therapeutic apheresis in childhood. J Clin Apher 1990; 5: 87–90.
Leibundgut K, Muller C, Muller K, Ridolfi-Luthy A, Hirt A . Tunneled, double lumen Broviac catheters are useful, efficient and safe in children undergoing peripheral blood progenitor cell harvesting and transplantation. Bone Marrow Transplant 1996; 17: 663–667.
Hengartner H, Berger C, Nadal D, Niggli FK, Grotzer MA . Port-A-Cath infections in children with cancer. Eur J Cancer 2004; 40: 2452–2458.
Munro FD, Gillett PM, Wratten JC, Schaw MP, Thomas A, Mac Kinlay GA et al. Totally implantable central venous access devices for paediatric oncology patients. Med Pediatr Oncol 1999; 33: 377–381.
O’Grady NP . Centre for Disease Control and Prevention. Guidelines for the Prevention of Intravascular Catheter-Related Infections. MMWR 2002; 51: 1–29.
Vardy J, Engelhardt K, Cox K, Jacquet J, Mc Dade A, Boyer M et al. Long-term outcome of radiological-guided insertion of implanted central venous access port devices (CVAPD) for the delivery of chemotherapy in cancer patients: institutional experience and review of the literature. Br J Cancer 2004; 91: 1045–1049.
Conter C, Carausu L, Martin E, Rubie H, Castex MP, Marec-Berard P . Central venous totally implantable access for high dose chemotherapy in children. Arch Pediatr 2006; 13: 256–261.
Rapport d’activité 2004. Agence de biomedecine. www.agence-biomedecine.fr/fr/rapport_2004. 2004.
Johansson E, Sollen Hansson A, Nilsson AS, Engervall P . Vascular access devices used during harvest of peripheral blood stem cells: high complication rate in patients with a long-term dialysis central venous catheter. Bone Marrow Transplant 1999; 24: 793–797.
Kanold J, Halle P, Rapatel C, Berger M, Gembara P, DeLumley L et al. Safe and efficient peripheral blood stem cell collection in the smallest of children. Ther Apher 1998; 2: 49–57.
Marson P, Petris MG, De Silvestro G . Collection of peripheral blood stem cells in pediatric patients: a concise review on technical aspects. Bone Marrow Transplant 1998; 22 (Suppl 5): S7–S11.
Croop JM, Cooper R, Seshadri R, Fernandez E, Graves V, Kreissmans S et al. Large-scale mobilization and isolation of CD34+ cells from normal donors. Bone Marrow Transplant 2000; 26: 1271–1279.
Alegre A, Requena MJ, Fernandez-Villalta MJ, Orts M, Gilsanz F, Tomas JF et al. Quinton-Mahurkar catheter as short-term central venous access for PBSC collection: single-center experience of 370 aphereses in 110 patients. Bone Marrow Transplant 1996; 18: 865–869.
Madero L, Diaz MA, Benito A, Villa M, Valdivielso A . Non-tunneled catheters for the collection and transplantation of peripheral blood stem cells in children. Bone Marrow Transplant 1997; 20: 53–56.
Haire WD, Lieberman RP, Lund GB, Wieczorek BM, Armitage JO, Kessinger A . Translumbar inferior vena cava catheters: experience with 58 catheters in peripheral stem cell collection and transplantation. Transfus Sci 1990; 11: 195–200.
Lazarus HM, Trehan S, Miller R, Fox RM, Creger RJ, Raaf JH . Multi-purpose silastic dual-lumen central venous catheters for both collection and transplantation of hematopoietic progenitor cells. Bone Marrow Transplant 2000; 25: 779–785.
Restrepo A, Devore P, Encarnacion CE, Wholey MH, Schneider D, Callander NS et al. Performance of a hybrid central venous catheter utilized for both peripheral blood stem cell harvest and transplant support of patients undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2002; 30: 389–395.
Biffi R, Pozzi S, Agazzi A, Pace U, Floridi A, Cenciarelli S et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol 2004; 15: 296–300.
Stroncek DF, Clay ME, Smith J, Jaszcz WB, Herr G, McCullough J . Comparison of two blood cell separators in collecting peripheral blood stem cell components. Transfus Med 1997; 7: 95–99.
Sarkodee-Adoo C, Taran I, Guo C, Buadi F, Murthy R, Cox E et al. Influence of preapheresis clinical factors on the efficiency of CD34+ cell collection by large-volume apheresis. Bone Marrow Transplant 2003; 31: 851–855.
Cecyn KZ, Seber A, Ginani VC, Goncalves AV, Caram EM, Oquro T et al. Large-volume leukapheresis for peripheral blood progenitor cell collection in low body weight pediatric patients: a single center experience. Transfus Apher Sci 2005; 32: 269–274.
Meisenberg BR, Callaghan M, Sloan C, Sampson L, Miller WE, McMillan R . Complications associated with central venous catheters used for the collection of peripheral blood progenitor cells to support high-dose chemotherapy and autologous stem cell rescue. Support Care Cancer 1997; 5: 223–227.
Moog R . Adverse events in peripheral progenitor cell collection: a 7-year experience. J Hematother Stem Cell Res 2001; 10: 675–680.
Perseghin P, Confalonieri G, Buscemi F, Dassi M, Poqliani E, Piotelli P et al. Electrolyte monitoring in patients undergoing peripheral blood stem cell collection. J Clin Apher 1999; 14: 14–17.
Schlenke P, Frohn C, Steinhardt MM, Kirchner H, Kluter H . Clinically relevant hypokalaemia, hypocalcaemia, and loss of hemoglobin and platelets during stem cell apheresis. J Clin Apher 2000; 15: 230–235.
Diaz MA, Kanold J, Vicent MG, Halle P, Madero L, Demeocq F . Using peripheral blood progenitor cells (PBPC) for transplantation in pediatric patients: a state-of-the-art review. Bone Marrow Transplant 2000; 26: 1291–1298.
Acknowledgements
We thank Marie-Dominique Reynaud for editorial assistance and nurses of the apheresis unit for their splendid work with the patients. We thank UBET for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carausu, L., Clapisson, G., Philip, I. et al. Use of totally implantable catheters for peripheral blood stem cell apheresis. Bone Marrow Transplant 40, 417–422 (2007). https://doi.org/10.1038/sj.bmt.1705756
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705756
Keywords
This article is cited by
-
Percutaneous retrieval of intravascular venous foreign bodies in children
Pediatric Radiology (2012)
-
Perforation of the superior vena cava after subclavian catheterization: a rare complication after autologous PBSCT
Bone Marrow Transplantation (2009)