Abstract
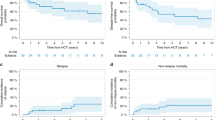
Allogeneic stem cell transplantation (ASCT) has improved leukemia-free survival (LFS) in many but not all patients with acute leukemia. This is an eight-year follow-up to our previous study showing a survival advantage to patients with an increased γδ T cells following ASCT. γδ T cell levels were collected prospectively in 153 patients (acute lymphoblastic leukemia (ALL) n=77; acute myelogenous leukemia (AML) n=76) undergoing partially mismatched related donor ASCT. Median age was 22 years (1–59), and 62% of the patients were in relapse at transplant. Patient–donor human leukocyte antigen (HLA) disparity of three antigens was 37% in the graft-versus-host disease (GvHD) and 29% in the rejection directions. All patients received a partially T cell-depleted graft using T10B9 (n=46) or OKT3 (n=107). Five years LFS and overall survival (OS) of patients with increased γδ compared to those with normal/decreased numbers were 54.4 vs 19.1%; P<0.0003, and 70.8 vs 19.6% P<0.0001, respectively, with no difference in GvHD (P=0.96). In a Cox multivariate analysis, normal/decreased γδ (hazard ratio (HR) 4.26, P=0.0002) and sex mismatch (HR 1.45 P=0.049) were associated with inferior LFS. In conclusion, γδ T cells may facilitate a graft-versus-leukemia (GvL) effect, without causing GvHD. Further evaluations of this effect may lead to specific immunotherapy for patients with refractory leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hessner MJ, Endean DJ, Casper JT, Horowitz MM, Keever-Taylor CA, Roth M et al. Use of unrelated marrow grafts compensates for reduced graft-versus-leukemia reactivity after T-cell-depleted allogeneic marrow transplantation for chronic myelogenous leukemia. Blood 1995; 86: 3987–3996.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol 1988; 69: 239–245.
Ho VT, Soiffer RJ . The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 2001; 98: 3192–3204.
Goldman JM, Gale RP, Horowitz MM, Biggs JC, Champlin RE, Gluckman E et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med 1988; 108: 806–814.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–2050.
Collins Jr RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Antin JH . Graft-versus-leukemia: no longer an epiphenomenon. Blood 1993; 82: 2273–2277.
Porter DL, Collins Jr RH, Shpilberg O, Drobyski WR, Connors JM, Sproles A et al. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant 1999; 5: 253–261.
Dazzi F, Goldman JM . Adoptive immunotherapy following allogeneic bone marrow transplantation. Annu Rev Med 1998; 49: 329–340.
Locatelli F . The role of repeat transplantation of haemopoietic stem cells and adoptive immunotherapy in treatment of leukaemia relapsing following allogeneic transplantation. Br J Haematol 1998; 102: 633–638.
Barrett AJ, Malkovska V . Graft-versus-leukaemia: understanding and using the alloimmune response to treat haematological malignancies. Br J Haematol 1996; 93: 754–761.
Truitt RL, Johnson BD . Principles of graft-vs-leukemia reactivity. Biol Blood Marrow Transplant 1995; 1: 61–68.
Porter DL, Antin JH . The graft-versus-leukemia effects of allogeneic cell therapy. Annu Rev Med 1999; 50: 369–386.
Bensussan A, Lagabrielle J, Degos L . TCR γδ bearing lymphocyte clones with lymphokine activated killer activity against autologous leukemic cells. Blood 1989; 73: 2077–2080.
Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 1989; 73: 1720–1728.
Ghayur T, Seemayer TA, Lapp WS . Kinetics of natural killer cell cytotoxicity during graft-versus-host reaction. Relationship between natural killer cell activity, T and B cell activity and development of histopathological alterations. Transplantation 1987; 44: 254.
Kolb HJ, Holler E . Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol 1997; 9: 139–145.
Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood 1996; 88: 2450–2457.
Pelot MR, Pearson DA, Swenson K, Zhao G, Sachs J, Yang YG et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based nonmyeloablative conditioning regimen. Biol Blood Marrow Transplant 1999; 5: 133–143.
Rhoades JL, Cibull ML, Thompson JS, Henslee-Downey PJ, Jennings CD, Sinn HP et al. Role of natural killer cells in the pathogenesis of human acute graft versus host disease. Transplantation 1993; 56: 113–120.
Slavin S, Ackerstein A, Morecki S, Gelfand Y, Cividalli G . Immunotherapy of relapsed resistant chronic myelogenous leukemia post allogeneic bone marrow transplantation with alloantigen pulsed donor lymphocytes. Bone Marrow Transplant 2001; 28: 795–798.
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294: 605–609.
Kaminski MJ, Cruz Jr PD, Bergstresser PR, Takashima A . Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res 1993; 53: 4014–4019.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Thomas S . Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 1999; 96: 6879–6884.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285: 727–729.
Groh V, Steinle A, Bauer S, Spies T . Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279: 1737–1740.
Boismenu R, Havran WL . An innate view of gamma delta T cells. Curr Opin Immunol 1997; 9: 57–63.
Lamb Jr LS, Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C et al. Increased frequency of TCR gamma delta+ T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother 1996; 5: 503–509.
Henslee-Downey PJ, Abhyankar SH, Parrish RS, Pati AR, Godder KT, Neglia WJ et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood 1997; 89: 3864–3872.
Godder KT, Hazlett LJ, Abhyankar SH, Chiang KY, Christiansen NP, Bridges KD et al. Partially mismatched related-donor bone marrow transplantation for pediatric patients with acute leukemia: younger donors and absence of peripheral blasts improve outcome. J Clin Oncol 2000; 18: 1856–1866.
Lee C, Brouillette M, Lamb L, Pati A, Brown S, Thompson J et al. Use of a closed system for V alpha beta-positive T cell depletion of marrow for use in partially mismatched related donor (PMRD) transplantation. Prog Clin Biol Res 1994; 389: 523–532.
Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee Fv et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant 2004; 33: 389–396.
Gray J . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Blazar BR, Taylor PA, Bluestone JA, Vallera DA . Murine gamma/delta-expressing T cells affect alloengraftment via the recognition of nonclassical major histocompatibility complex class Ib antigens. Blood 1996; 87: 4463–4472.
Kawanishi Y, Passweg J, Drobyski WR, Rowlings P, Cook-Craig A, Casper J et al. Effect of T cell subset dose on outcome of T cell-depleted bone marrow transplantation. Bone Marrow Transplant 1997; 19: 1069–1077.
Bigby M, Markowitz JS, Bleicher PA, Grusby MJ, Simha S, Siebrecht M et al. Most gamma delta T cells develop normally in the absence of MHC class II molecules. J Immunol 1993; 151: 4465–4475.
Lamb Jr LS, Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ et al. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant 2001; 27: 601–606.
Norton J, al-Saffar N, Sloane JP . An immunohistological study of gamma/delta lymphocytes in human cutaneous graft-versus-host disease. Bone Marrow Transplant 1991; 7: 205–208.
Drobyski WR, Majewski D, Hanson G . Graft-facilitating doses of ex vivo activated gammadelta T cells do not cause lethal murine graft-vs-host disease. Biol Blood Marrow Transplant 1999; 5: 222–230.
Drobyski WR, Vodanovic-Jankovic S, Klein J . Adoptively transferred gamma delta T cells indirectly regulate murine graft-versus-host reactivity following donor leukocyte infusion therapy in mice. J Immunol 2000; 165: 1634–1640.
Ellison CA, MacDonald GC, Rector ES, Gartner JG . Gamma delta T cells in the pathobiology of murine acute graft-versus-host disease. Evidence that gamma delta T cells mediate natural killer-like cytotoxicity in the host and that elimination of these cells from donors significantly reduces mortality. J Immunol 1995; 155: 4189–4198.
Schilbach KE, Geiselhart A, Wessels J, TNiethammer D, Handgretingee R . Human gammadelta T lymphocytes exert natural and IL-2-induced cytotoxicity to neuroblastoma cells. J Immunother 2000; 23: 536–548.
Cela ME, Holladay MS, Rooney CM, Richardson S, Alexander B, Krance RA et al. Gamma delta T lymphocyte regeneration after T lymphocyte-depleted bone marrow transplantation from mismatched family members or matched unrelated donors. Bone Marrow Transplant 1996; 17: 243–247.
Yabe M, Yabe H, Hattori K, Hinohara T, Morimoto T, Kato S et al. Transition of T cell receptor gamma/delta expressing double negative (CD4-/CD8-) lymphocytes after allogeneic bone marrow transplantation. Bone Marrow Transplant 1994; 14: 741–746.
Viale M, Ferrini S, Bacigalupo A . TCR gamma/delta positive lymphocytes after allogeneic bone marrow transplantation. Bone Marrow Transplant 1992; 10: 249–253.
Tsuji S, Char D, Bucy RP, Simonsen M, Chen CH, Cooper MD . Gamma delta T cells are secondary participants in acute graft-versus-host reactions initiated by CD4+ alpha beta T cells. Eur J Immunol 1996; 26: 420–427.
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102: 200–206.
Meeh P, King KM, O'Brien R, Muga S, Buckhalts P, Neuberg R et al. Characterization of the γδ T cell response to acute leukemia. Cancer Immunol Immunother 2006; 55: 1072–1080.
Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AF, Torres M et al. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol 1998; 161: 1558–1567.
Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G et al. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol 2003; 120: 829–834.
Ferrarini M, Heltai S, Toninelli E, Sabbadini MG, Pellicciari C, Manfredi AA . Daudi lymphoma killing triggers the programmed death of cytotoxic V gamma 9/V delta 2T lymphocytes. J Immunol 1995; 154: 3704–3712.
Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M . Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000; 96: 384–392.
Lamb Jr LS, Lopez RD . gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant 2005; 11: 161–168.
Steinle A, Groh V, Spies T . Diversification, expression, and gamma delta T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. ProcNatl Acad Sci USA 1998; 95: 12510–12515.
Dolstra H, Fredrix H, van der Meer A, de Witte T, Figdor C, van de Wiel-van et al. TCR gamma delta cytotoxic T lymphocytes expressing the killer cell-inhibitory receptor p58.2 (CD158b) selectively lyse acute myeloid leukemia cells. Bone Marrow Transplant 2001; 27: 1087–1093.
Duval M, Yotnda P, Bensussan A, Oudhiri N, Guidal C, Rohrlich P et al. Potential antileukemic effect of gamma delta T cells in acute lymphoblastic leukemia. Leukemia 1995; 9: 863–868.
Keever CA, Small TN, Flomenberg N, Heller G, Pekle K, Black P et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood 1989; 73: 1340–1350.
Foot AB, Potter MN, Donaldson C, Cornish JM, Wallington TB, Oakhill A et al. Immune reconstitution after BMT in children. Bone Marrow Transplant 1993; 11: 7–13.
Kook H, Goldman F, Giller R, Goeken N, Peters C, Comito M et al. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: immunophenotypic analysis and factors affecting the speed of recovery. Blood 1996; 88: 1089–1097.
Lamb LS HL, Gee A, Hazlett L, Musk P, Parrish R, O'Hanlon T et al. Influence of T cell depletion method on circulating γδ+ T cell reconstitution and potential role in the graft-versus-leukemia effect. Cytotherapy 1999; 1: 7–19.
Acknowledgements
This study was supported by NIH NCI R21 CA 76667 and Leukemia and Lymphoma Society 6507. The study was presented in part at the ASH meeting 2002 and ASCO meeting 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Godder, K., Henslee-Downey, P., Mehta, J. et al. Long term disease-free survival in acute leukemia patients recovering with increased γδ T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant 39, 751–757 (2007). https://doi.org/10.1038/sj.bmt.1705650
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705650
Keywords
This article is cited by
-
Automatic generation of alloreactivity-reduced donor lymphocytes and hematopoietic stem cells from the same mobilized apheresis product
Journal of Translational Medicine (2023)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Improved Vδ2+ T cells recovery correlates to reduced incidences of mortality and relapse in acute myeloid leukemia after hematopoietic transplantation
Annals of Hematology (2023)
-
Expansions of tumor-reactive Vdelta1 gamma-delta T cells in newly diagnosed patients with chronic myeloid leukemia
Cancer Immunology, Immunotherapy (2023)
-
Treatment of adult ALL patients with third-generation CD19-directed CAR T cells: results of a pivotal trial
Journal of Hematology & Oncology (2023)