Abstract
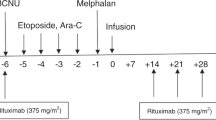
The potential benefit of rituximab as adjuvant to high-dose therapy (HDT) has been investigated in patients under 60 years with poor-risk (age-adjusted international prognostic index at 2–3) CD20+ diffuse large B-cell lymphoma (DLBCL). The treatment consisted of four cycles of high-dose CEOP (cyclophosphamide, epirubicin, vincristine, prednisone), plus etoposide and cisplatin during the two last cycles. Peripheral blood stem cells were collected after cycle 1, and reinfused after cycles 3 and 4. Four weekly rituximab infusions were subsequently delivered. Among the 36 patients included, 30 could complete chemotherapy schedule, and 24/36 received rituximab. A complete response occured in 26/36 patients (72%). With a median follow-up of 30 months, the estimated 5-year overall survival (OS) and event-free survival (EFS) rates (mean±s.d.) were 65±16 and 63±15%, respectively. For the 24 patients who received both chemotherapy and rituximab, the estimated 5-year OS and EFS rates were 86±14 and 82±15%. These data suggest that rituximab after HDT is feasible. Both complete remission rate and survival curves compare favorably with the poor outcome usually observed in high-risk DLBCL patients managed with HDT without rituximab.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting – Airlie House, Virginia, November, 1997. Hematol J 2000; 1: 53–66.
The international non-Hodgkin's lymphoma prognostic factors project: a predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993; 329: 987–994.
Haioun C, Lepage E, Gisselbrecht C, Bastion Y, Coiffier B, Brice P et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non Hodgkin's lymphoma: Updated results of the prospective study LNH87-2. J Clin Oncol 1997; 15: 1131–1137.
Santini G, Salvagno L, Leoni P, Chisesi T, De Souza C, Sertoli MR et al. VACOP-B versus VACOP-B plus autologous bone marrow transplantation for advanced diffuse non Hodgkin's lymphoma: Results of a prospective randomized trial by the non Hodgkin's lymphoma cooperative study group. J Clin Oncol 1998; 16: 2796–2802.
Haioun C, Lepage E, Gisselbrecht C, Thieblemont C, Simon D, Rose C et al. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol – A Groupe d'Etude des Lymphomes de l'Adulte Study. J Clin Oncol 2000; 18: 3025–3030.
Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med 2004; 350: 1287–1295.
Shipp MA, Neuberg D, Janicck M, Canellos GP, Shulman LN . High-dose CHOP as initial therapy for patients with poor-prognosis aggressive non Hodgkin's lymphoma. A dose-finding pilot study. J Clin Oncol 1995; 13: 2916–2923.
Gianni AM, Bregni M, Siena S, Brambilla C, Di Nicola M, Lombardi F et al. High-dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med 1997; 336: 1290–1297.
Stoppa AM, Bouabdallah R, Chabannon C, Novakovitch G, Vey N, Camerlo J et al. Intensive sequential chemotherapy with repeated blood stem-cell support for untreated poor-prognosis non Hodgkin's lymphoma. J Clin Oncol 1997; 15: 1722–1729.
Bouabdallah R, Stoppa AM, Rossi JF, Lepeu G, Coso D, Xerri L et al. Intensive sequential chemotherapy (ISC 95) with growth factors and blood stem cell support in high-intermediate and highrisk (IPI 2 and IPI 3) aggressive non Hodgkin's lymphoma: an oligocentric report on 42 patients. Leukemia 1999; 13: 950–956.
Bouabdallah R, Stoppa AM, Coso D, Bardou VJ, Blaise D, Chabannon C et al. Clinical outcome after front-line intensive sequential chemotherapy (ISC) in patients with aggressive non-Hodgkin's lymphoma and high-risk international prognostic index (IPI 3): final analysis of survival in two consecutive ISC trials. Ann Oncol 2001; 12: 513–517.
Greb A, Schiefer DH, Bohlius J, Schwarzer G, Engert A . High-dose chemotherapy with autologous stem cell support is not superior to conventional-dose chemotherapy in the first-line treatment of aggressive non-Hodgkin's lymphoma – results of a comprehensive meta-analysis. Blood 2004; 104 (abstract # 920).
Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 1998; 92: 1927–1932.
Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med 2002; 346: 235–242.
Habermann TM, Weller E, Morrison VA, Cassileth PA, Cohn J, Dakhil S et al. Rituximab–CHOP versus CHOP with or without maintenance rituximab in patients 60 years of age or older with diffuse large B-cell lymphoma: an update. Blood 2004; 104 (abstract # 127).
Pfreundschuh M, Truemper L, Gill D, Osterborg A, Pettengell R, Trneny M et al. First analysis of the completed Mabhtera International Trial (MinT) in young patients with low-risk diffuse large B-cell lymphoma (DLBCL): addition of rituximab to a CHOP-like regimen significantly improves outcome of all patients with the identification of a very favourable subgroup with IPI=0 and no bulky disease. Blood 2004; 104 (abstract # 157).
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 1999; 17: 1244–1253.
Horwitz SM, Negrin RS, Blume KG, Breslin S, Stuart MJ, Stockerl-Goldstein KE et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood 2004; 103: 777–783.
Zanni M, Di Nicola M, Patti C, Castellino C, Pescarollo A, Zoli V et al. Rituximab-supplemented high-dose sequential (HDS) chemotherapy with autograft is highly effective in high-risk (aaIPI 2-3) diffuse large B-cell lymphoma: Results of a prospective multicenter study on 91 consecutive patients treated at diagnosis. Blood 2004; 104 (abstract # 891).
Milpied NJ, Lamy T, Casassus P, Deconninck E, Gressin R, Maisonneuve H et al. Front-line high-dose chemotherapy (HDT) combined with rituximab for adults with aggressive large B-cell lymphoma (DLBCL): GOELAMS 074 trial. Blood 2004; 104 (abstract # 902).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coso, D., Sebban, C., Boulat, O. et al. A phase II trial of rituximab as adjuvant to intensive sequential chemotherapy in patients under 60 years with untreated poor-prognosis diffuse large B-cell lymphoma. Bone Marrow Transplant 38, 217–222 (2006). https://doi.org/10.1038/sj.bmt.1705414
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705414