Abstract
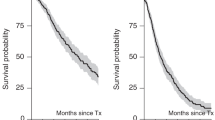
Although high-dose therapy and autologous stem cell transplant (ASCT) is superior to conventional chemotherapy for treatment of myeloma, most patients relapse and the time to relapse depends upon the initial prognostic factors. The administration of non-cross-resistant chemotherapies during the post-transplant period may delay or prevent relapse. We prospectively studied the role of consolidation chemotherapy (CC) after single autologous peripheral blood stem cell transplant (auto-PBSCT) in 103 mostly newly diagnosed myeloma patients (67 patients were ⩽6 months from the initial treatment). Patients received conditioning with BCNU, melphalan±gemcitabine and auto-PBSCT followed by two cycles of the DCEP±G regimen (dexamethasone, cyclophosphamide, etoposide, cisplatin±gemcitabine) at 3 and 9 months post-transplant and alternating with two cycles of DPP regimen (dexamethasone, cisplatin, paclitaxel) at 6 and 12 months post-transplant. With a median follow-up of 61.2 months, the median event-free survival (EFS) and overall survival (OS) are 26 and 54.1 months, respectively. The 5-year EFS and OS are 23.1 and 42.5%, respectively. Overall, 51 (49.5%) patients finished all CC, suggesting that a major limitation of this approach is an inability to deliver all planned treatments. In order to improve results following autotransplantation, novel agents or immunologic approaches should be studied in the post-transplant setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Harousseau JL, Shaughnessy Jr J, Richardson P . Multiple myeloma. Hematology (Am Soc Hematol Educ Program) 2004, 237–256.
Barille-Nion S, Barlogie B, Bataille R, Bergsagel PL, Epstein J, Fenton RG et al. Advances in biology and therapy of multiple myeloma. Hematology (Am Soc Hematol Educ Program) 2003, 248–278.
Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E et al. Treatment of multiple myeloma. Blood 2004; 103: 20–32.
Kyle RA, Rajkumar SV . Multiple myeloma. N Engl J Med 2004; 351: 1860–1873.
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 2003; 349: 2495–2502.
Munshi NC, Desikan KR, Jagannath S, Siegel D, Bracy D, Tricot G et al. Dexamethasone, cyclophosphamide, etoposide and cis-platinum (DCEP), an effective regimen for relapse after high-dose chemotherapy and autologous transplantation (AT). Blood 1996; 2331: 586a.
Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace Jr AJ, Hohn KW et al. An information-intensive approach to the molecular pharmacology of cancer. Science 1997; 275: 343–349.
Drach J, Ackermann J, Fritz E, Kromer E, Schuster R, Gisslinger H et al. Presence of a p53 gene deletion in patients with multiple myeloma predicts for short survival after conventional-dose chemotherapy. Blood 1998; 92: 802–809.
Haldar S, Jena N, Croce CM . Antiapoptosis potential of bcl-2 oncogene by dephosphorylation. Biochem Cell Biol 1994; 72: 455–462.
Miguel-Garcia A, Orero T, Matutes E, Carbonell F, Miguel-Sosa A, Linares M et al. Bcl-2 expression in plasma cells from neoplastic gammopathies and reactive plasmacytosis: a comparative study. Haematologica 1998; 83: 298–304.
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–1123.
Gojo I, Guo C, Sarkodee-Adoo C, Meisenberg B, Fassas A, Rapoport AP et al. High-dose cyclophosphamide with or without etoposide for mobilization of peripheral blood progenitor cells in patients with multiple myeloma: efficacy and toxicity. Bone Marrow Transplant 2004; 34: 69–76.
Fassas AB, Spencer T, Desikan R, Zangari M, Anaissie E, Barlogie B et al. Cytotoxic chemotherapy following tandem autotransplants in multiple myeloma patients. Br J Haematol 2002; 119: 164–168.
Rapoport AP, Guo C, Badros A, Hakimian R, Akpek G, Kiggundu E et al. Autologous stem cell transplantation followed by consolidation chemotherapy for relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplant 2004; 34: 883–890.
Rapoport AP, Meisenberg B, Sarkodee-Adoo C, Fassas A, Frankel SR, Mookerjee B et al. Autotransplantation for advanced lymphoma and Hodgkin's disease followed by post-transplant rituxan/GM-CSF or radiotherapy and consolidation chemotherapy. Bone Marrow Transplant 2002; 29: 303–312.
Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 1999; 93: 55–65.
Alexanian R, Weber D, Giralt S, Dimopoulos M, Delasalle K, Smith T et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant 2001; 27: 1037–1043.
Davies FE, Forsyth PD, Rawstron AC, Owen RG, Pratt G, Evans PA et al. The impact of attaining a minimal disease state after high-dose melphalan and autologous transplantation for multiple myeloma. Br J Haematol 2001; 112: 814–819.
Lahuerta JJ, Martinez-Lopez J, Serna JD, Blade J, Grande C, Alegre A et al. Remission status defined by immunofixation vs electrophoresis after autologous transplantation has a major impact on the outcome of multiple myeloma patients. Br J Haematol 2000; 109: 438–446.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood 2002; 99: 731–735.
Stewart AK, Chen CI, Howson-Jan K, White D, Roy J, Kovacs MJ et al. Results of a multicenter randomized phase II trial of thalidomide and prednisone maintenance therapy for multiple myeloma after autologous stem cell transplant. Clin Cancer Res 2004; 10: 8170–8176.
Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 2004; 127: 165–172.
Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol 2002; 20: 4319–4323.
Richardson P, Anderson K . Immunomodulatory analogs of thalidomide: an emerging new therapy in myeloma. J Clin Oncol 2004; 22: 3212–3214.
Richardson P, Schlossman R, Jagannath S, Alsina M, Desikan R, Blood E et al. Thalidomide for patients with relapsed multiple myeloma after high-dose chemotherapy and stem cell transplantation: results of an open-label multicenter phase 2 study of efficacy, toxicity, and biological activity. Mayo Clin Proc 2004; 79: 875–882.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–2617.
Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood 2002; 100: 3063–3067.
Barlogie B . Thalidomide and CC-5013 in multiple myeloma: the University of Arkansas experience. Semin Hematol 2003; 40: 33–38.
Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood 2002; 100: 3919–3924.
Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood 2003; 102: 3447–3454.
Borrello I, Bierdrzycki B, Sheets N, George B, Racke F, Loper K et al. Autologous tumor combined with a GM-CSF-secreting cell line vaccine (GVAX) following autologous stem cell transplant (ASCT) in multiple myeloma. Blood 2004; 440: 126a.
Rapoport A, Stadtmauer EA, Levine BL, Badros A, Akpek G, Cotte J et al. Adoptive transfer of ex vivo costimulated autologous T-cells in conjuction with pneumococcal conjugate vaccine immunizations accelerates post-transplant T-cell recovery after autotransplantation for myeloma: results of a randomized study. Blood 2004; 439: 126a.
Acknowledgements
We thank the nurses of the BMT unit for outstanding patient care. APR is a Clinical Scholar of the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gojo, I., Meisenberg, B., Guo, C. et al. Autologous stem cell transplantation followed by consolidation chemotherapy for patients with multiple myeloma. Bone Marrow Transplant 37, 65–72 (2006). https://doi.org/10.1038/sj.bmt.1705192
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705192
Keywords
This article is cited by
-
High-dose chemotherapy and autologous hematopoietic stem cell transplantation in myeloma patients under the age of 65 years
Bone Marrow Transplantation (2007)