Summary:
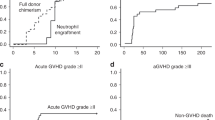
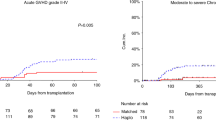
Alemtuzumab is a humanized monoclonal antibody directed against human CD52 with a strong lympholytic effect. We have performed unmanipulated hematopoietic stem cell transplantation (HSCT) from 2- or 3-locus-mismatched family donors in 14 patients using in vivo alemtuzumab. All achieved complete donor cell engraftment and grade III–IV acute graft-versus-host disease was observed in only one patient. However, eight of the 14 patients developed grade II–IV cardiac complications according to Bearman's criteria. Next, we retrospectively analyzed the records of 142 adult patients who underwent allogeneic HSCT from 1995 to 2004 to evaluate whether the use of alemtuzumab was an independent risk factor for cardiac complications. Among several factors that increased the incidence of grade II–IV cardiac complications with at least borderline significance, a multivariate analysis identified the cumulative dose of anthracyclines (P=0.0016) and the use of alemtuzumab (P=0.0001) as independent significant risk factors. All of the cardiac complications in the alemtuzumab group were successfully treated with diuretics and/or catecholamines. Patient selection and close monitoring of cardiac function may be important in HLA-mismatched HSCT using in vivo alemtuzumab.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hale G . The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy 2001; 3: 137–143.
Chakraverty R, Peggs K, Chopra R et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood 2002; 99: 1071–1078.
Kottaridis PD, Milligan DW, Chopra R et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Faulkner RD, Craddock C, Byrne JL et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood 2004; 103: 428–434.
Ho AY, Pagliuca A, Kenyon M et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood 2004; 104: 1616–1623.
Peggs KS, Mackinnon S, Williams CD et al. Reduced-intensity transplantation with in vivo T-cell depletion and adjuvant dose-escalating donor lymphocyte infusions for chemotherapy-sensitive myeloma: limited efficacy of graft-versus-tumor activity. Biol Blood Marrow Transplant 2003; 9: 257–265.
Kanda Y, Oshima K, Asano-Mori Y et al. In vivo alemtuzumab enables haploidentical HLA-mismatched hematopoietic stem cell transplantation without ex vivo graft manipulation. Transplantation 2005; 79: 1351–1357.
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Sakata-Yanagimoto M, Kanda Y, Nakagawa M et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant 2004; 33: 1043–1047.
Ferrajoli A, O'Brien SM, Cortes JE et al. Phase II study of alemtuzumab in chronic lymphoproliferative disorders. Cancer 2003; 98: 773–778.
Kennedy GA, Seymour JF, Wolf M et al. Treatment of patients with advanced mycosis fungoides and Sezary syndrome with alemtuzumab. Eur J Haematol 2003; 71: 250–256.
Dearden CE, Matutes E, Cazin B et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood 2001; 98: 1721–1726.
Pawson R, Dyer MJ, Barge R et al. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol 1997; 15: 2667–2672.
Lenihan DJ, Alencar AJ, Yang D et al. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome. Blood 2004; 104: 655–658.
Mann DL . Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 2002; 91: 988–998.
Blum A, Miller H . Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med 2001; 52: 15–27.
Spitzer TR . Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 893–898.
Niwa N, Watanabe E, Hamaguchi M et al. Early and late elevation of plasma atrial and brain natriuretic peptides in patients after bone marrow transplantation. Ann Hematol 2001; 80: 460–465.
Acknowledgements
This research was supported in part by grants from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshima, K., Sakata-Yanagimoto, M., Asano-Mori, Y. et al. Cardiac complications after haploidentical HLA-mismatched hematopoietic stem cell transplantation using in vivo alemtuzumab. Bone Marrow Transplant 36, 821–824 (2005). https://doi.org/10.1038/sj.bmt.1705145
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705145
Keywords
This article is cited by
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)