Summary:
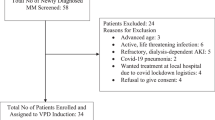
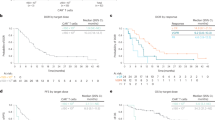
Interleukin-6 (IL-6) is a major survival factor for multiple myeloma (MM) cells preventing apoptosis induced by dexamethasone (DEX) or chemotherapy. In all, 24 consecutive patients with MM in first-line therapy received DEX for 4 days, followed by melphalan (HDM: 140 mg/m2) and autologous stem cell transplantation (ASCT). The anti-IL-6 monoclonal antibody (mAb) (B-E8) was given till haematological recovery, starting 1 day before DEX. Results were historically compared to MM patients treated with HDM 140 and 200 mg/m2. Our results show (1) that B-E8 was able to fully neutralize IL-6 activity in vivo before and after HDM as shown by inhibition of C reactive protein (CRP) production; (2) no haematological toxicity; (3) a significant reduction of mucositis and fever; (4) a median event-free survival of 35 months and an overall survival of 68.2% at 5 years with a median follow-up of 72 months; and (5) the overall daily IL-6 production progressively increased on and after 7 days post-HDM, with the increased serum CRP levels. In the 5/24 patients with uncontrolled CRP production, a large IL-6 production was detected (320 μg/day) that could not possibly be neutralized by B-E8. These data show the feasibility to neutralize IL-6 in vivo with anti-IL-6 mAb in the context of HDM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bataille R, Harousseau JL . Multiple myeloma. N Engl J Med 1997; 336: 1657–1664.
Attal M, Harousseau JL, Stoppa AM et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 1996; 335: 91–97.
Lenhoff S, Hjorth M, Holmberg E et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood 2000; 95: 7–11.
Attal M, Harousseau JL, Facon T et al. InterGroupe Francophone du Myelome. single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 2003; 349: 2495–2502.
Moreau P, Facon T, Attal M et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma final: analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood 2002; 99: 731–735.
Sieghel DS, Desikan KR, Mehta J et al. Age is not a prognostic variable with autologous transplantation for multiple myeloma. Blood 1999; 93: 51–54.
Barlogie B, Jagannath S, Vesole DH et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood 1997; 89: 789–793.
Barlogie B, Jagannath S, Desikan KR et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 1999; 93: 55–65.
Goldschmidt H, Egerer G, Ho AD . Autologous and allogenic stem cell transplantation in multiple myeloma. Bone Marrow Transplant 2000; 25 (Suppl 2): S25–S26.
Rossi JF, Legouffe E, Fegueux N et al. Autologous transplantation (AT) of CD34+ peripheral blood progenitor cells (PBPC) after double (D) high dose chemotherapy (HDC) in multiple myeloma (MM) is followed by severe immunodeficiency (ID) and high production of interleukin-6 (IL-6) related-C Reactive Protein (CRP) requiring additive immunotherapy (IT). Blood 1996; 88 (Suppt1): 132a (abstract 516).
Lemoli RM, Martinelli G, Zamagni E et al. Engraftment, clinical, and molecular follow-up of patients with multiple myeloma who were reinfused with highly purified CD34+ cells to support single or tandem high-dose chemotherapy. Blood 2000; 95: 2234–2239.
Stewart AK, Vescio R, Schiller G et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol 2001; 19: 3771–3779.
Kawano M, Hirano T, Matsuda T et al. Autocrine generation and essential requirement of BSF-2/IL-6 for human multiple myeloma. Nature 1988; 332: 83–87.
Klein B, Zhang XG, Lu ZY, Bataille R . Interleukin-6 in multiple myeloma. Blood 1995; 85: 863–872.
Klein B, Zhang XG, Jourdan M et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood 1989; 73: 517–526.
Klein B, Zhang XG, Jourdan M et al. Interleukin-6 is the central tumor growth factor in vitro and in vivo in multiple myeloma. Eur Cytokine Netw 1990; 1: 193–201.
Zhang XG, Gaillard JP, Robillard N et al. Reproducible obtaining myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood 1994; 83: 3654–3663.
Portier M, Rajzbaum G, Zhang XG et al. In vivo interleukin 6 gene expression in the tumoral environment in multiple myeloma. Eur J Immunol 1991; 21: 1759–1762.
Greipp P, Leong T, Benett JM et al. Plasmablastic morphology, an independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) myeloma trial E 9486 report by the ECOG Myeloma Laboratory Group. Blood 1998; 91: 2501–2507.
Stasi R, Brunetti M, Parma A et al. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer 1998; 82: 1860–1866.
Klein B, Widjenes J, Zhang XG et al. Murine anti-IL-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood 1991; 78: 1198–1204.
Bataille R, Barlogie B, Lu ZY et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood 1995; 86: 685–691.
Lu ZY, Brailly H, Rossi JF et al. Overall interleukin-6 production exceeds 7 mg/day in multiple myeloma complicated by sepsis. Cytokine 1993; 5: 578–582.
Lu ZY, Brailly H, Wijdenes J et al. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood 1995; 86: 3124–3131.
Blay JY, Rossi JF, Widjenes J, Menetrier-Caux C . Role of interleukin-6 in the paraneoplastic syndrome associated with renal cell carcinoma. Int J Cancer 1997; 72: 424–430.
Frassanito MA, Cussmai A, Iodice G, Dammacco F . Autocrine interleukin-6 production and highly malignant multiple myeloma: relation with resistance to drug-induced apoptosis. Blood 2001; 97: 483–489.
Borsellino N, Belldegrun A, Bonavida B . Endogenous interleukin-6 is a resistance factor for cis-diamminedichloroplatinum and etoposide-mediated cytotoxicity of human prostate carcinoma cell lines. Cancer Res 1995; 55: 4633–4639.
Mizutani Y, Bonavida B, Koishihara Y et al. Sensitization of human renal cell carcinoma cells to cis-diamminedichloroplatinum(II) by anti-interleukin 6 monoclonal antibody or anti-interleukin 6 receptor monoclonal antibody. Cancer Res 1995; 55: 590–596.
Klein B, Brailly H . Cytokine-binding proteins: stimulating antagonists. Immunol Today 1995; 16: 216–220.
Montero-Julian F, Klein B, Gautherot E, Brailly H . Pharmacokinetic study of anti-interleukin-6 (IL-6) therapy with monoclonal antibodies: enhancement of IL-6 clearance by cocktails of anti-IL-6 antibodies. Blood 1995; 85: 917–924.
Blade J, Samson D, Reece D et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated with high-dose therapy and haematopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European bone Marrow Transplant. Br J Haematol 1998; 102: 1115–1123.
Widjenes J, Clement C, Klein B et al. Human recombinant dimeric IL-6 binds to its receptor as detected by anti-IL-6 monoclonal antibodies. Mol Immunol 1991; 28: 1183–1192.
Kaplan EL, Meier P . Non parametric estimations from incomplete observations. J Am Stat Assoc 1958; 53: 457.
Steffen M, Durken M, Pichlmeier U et al. Serum interleukin-6 levels during bone marrow transplantation: impact on transplant-related toxicity and engraftment. Bone Marrow Transplant 1996; 18: 301–307.
Veldhuis GJ, Willemse PH, Sleijfer DT et al. Toxicity and efficacy of escalating dosages of recombinant human interleukin-6 after chemotherapy in patients with breast cancer or non-small-cell lung cancer. J Clin Oncol 1995; 13: 2585–2593.
Trickha M, Corringham R, Klein B, Rossi JF . Targetted anti-interleukin-6 monoclonal antibody therapy for cancer: review of the rationale and clinical evidence. Clin Cancer Res 2003; 9: 4653–4665.
Beck JT, Hsu SM, Wijdenes J et al. Brief report: alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med 1994; 330: 602–605.
Emilie D, Widjenes J, Gisselbrecht C et al. Administration of an anti-interleukin-6 monoclonal antibody to patients with acquired immunodeficiency syndrome and lymphoma: effects on lymphoma growth and on B clinical symptoms. Blood 1994; 84: 2472–2479.
Legouffe E, Liautard J, Gaillard JP et al. Human anti-mouse antibody response to the injection of murine monoclonal antibodies against IL-6. Clin Exp Immunol 1994; 98: 323–329.
Castell JV, Gomez-Lechon MJ, David M et al. Acute phase response of human hepatocytes: regulation of acute phase protein synthesis by interleukin-6. Hepatology 1990; 12: 1179–1186.
Kollet O, Aviram R, Chebath J . The soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro maintenance and proliferation of human CD34+CD38-/low cells capable of repopulating severe combined immunodeficiency mice. Blood 1999; 94: 923–931.
Sun L, Liu X, Qiu L et al. Administration of plasmid DNA expressing human interleukin-6 significantly improves thrombocytopoiesis in irradiated mice. Ann Hematol 2001; 80: 567–572.
Kaser A, Brandacher G, Steurer W et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 2001; 98: 2720–2725.
Efferth T, Fabry U, Osieka R . Interleukin-6 affects melphalan-induced DNA damage and repair in human multiple myeloma cells. Anticancer Res 2002; 22: 231–234.
Rowley M, Liu P, Van Ness B . Heterogeneity in therapeutic response of genetically altered myeloma cell lines to interleukin-6, dexamethasone, doxorubicin and melphalan. Blood 2000; 96: 3175–3180.
Tegg EM, Griffiths AE, Lowenthal RM et al. Association between high interleukin-6 levels and adverse outcome after autologous haemopoietic stem cell transplantation. Bone Marrow Transplant 2001; 28: 929–933.
Moreau P, Milpied N, Mahé B et al. 220 mg/m2 melphalan followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant 1999; 23: 1003–1006.
Durie BGM, Salmon SE . A critical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975; 36: 842–854.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, JF., Fegueux, N., Lu, Z. et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant 36, 771–779 (2005). https://doi.org/10.1038/sj.bmt.1705138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705138
Keywords
This article is cited by
-
Mesenchymal stromal cell senescence in haematological malignancies
Cancer and Metastasis Reviews (2023)
-
Immune precision medicine for cancer: a novel insight based on the efficiency of immune effector cells
Cancer Communications (2019)
-
Elevated pre-transplant C-reactive protein identifies a high-risk subgroup in multiple myeloma patients undergoing delayed autologous stem cell transplantation
Bone Marrow Transplantation (2018)
-
Tumor-related interleukins: old validated targets for new anti-cancer drug development
Molecular Cancer (2017)
-
URI regulates tumorigenicity and chemotherapeutic resistance of multiple myeloma by modulating IL-6 transcription
Cell Death & Disease (2014)