Summary:
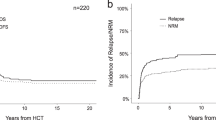
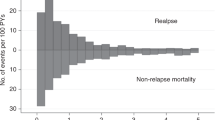
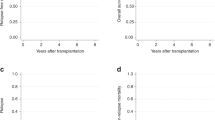
The major cause of failure after allogeneic hematopoietic stem cell transplantation (HSCT) for acute myelogenous leukemia (AML) is disease relapse or progression. We analyzed the outcome of second HSCT for treatment of patients with relapsed, refractory AML/myelodysplastic syndrome (MDS) at our institution. A total of 72 patients were eligible for this analysis. In all, 25 (35%) patients received salvage chemotherapy prior to the second transplant procedure and only two (3%) patients were in complete remission at the time of the second transplant. A total of 20 patients (28%) had low leukemia burden as measured by the absence of peripheral blood blasts and ⩽5% blasts in the bone marrow at the time of the second transplant. Although, the overall median survival after the second transplant was 6 months, a subset of patients who had low leukemia burden at the time of the second transplant had a 5-year survival of 25 vs 12% in those with a high leukemia burden. Thus, a second transplant may offer the possibility of long-term disease control in a subset of patients who have a ‘low bulk’ disease at the time of transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Frassoni F, Barrett A, Granena A et al. Relapse after allogeneic bone marrow transplantation for acute leukemia: a survey by the EBMT of 117 cases. Br J Haematol 1988; 70: 317–320.
Collins R, Shpilberg O, Drobyski W et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Bacigalupo A, Soracco M, Vassallo F et al. Donor lymphocyte infusion (DLI) in patients with chronic myeloid leukemia following allogeneic bone marrow transplantation. Bone Marrow Transplant 1997; 19: 927–932.
Kolb H, Schattenberg A, Goldman J et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients: European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood 1995; 86: 2041–2050.
Radich JP, Gooley T, Sanders JE et al. Second allogeneic transplantation after failure of first autologous transplantation. Biol Blood Marrow Transplant 2000; 6: 272–279.
Bosi A, Laszlo D, Labopin M, et al, for the Acute Leukemia Working Party of the European Blood and Marrow Transplant Group. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol 2001; 19: 3675–3684.
Przepiorka D, Weisdorf D, Martin P et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Wilcoxon F . Individual comparisons by ranking methods. Biometrics 1945; 1: 80–83.
Stata Corporation. StataCorp: Stata Statistical Software: Release 7.0. Stata Corporation: College Station, TX, 2001.
Champlin R, Khouri I, Shimoni A et al. Harnessing graft versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol 2000; 111: 18–29.
Andersson BS, Kashyap A, Gian V et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 2002; 8: 145–154.
Clift RA, Buckner CD, Thomas ED et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood 1994; 84: 2036–2043.
Ravandi F, Kantarjian H, Cohen A et al. Decitabine with allogeneic peripheral blood stem cell transplantation in the therapy of leukemia relapse following a prior transplant: results of a phase I study. Bone Marrow Transplant 2001; 27: 1221–1225.
de Lima M, van Besien K, Gajewski J et al. High-dose melphalan and allogeneic peripheral blood stem cell transplantation for treatment of early relapse after allogeneic transplant. Bone Marrow Transplant 2000; 26: 333–338.
de Lima M, Couriel D, Thall PF et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Bibawi S, Abi-Said D, Fayad L et al. Thiotepa, busulfan, and cyclophosphamide as a preparative regimen for allogeneic transplantation for advanced myelodysplastic syndrome and acute myelogenous leukemia. Am J Hematol 2001; 67: 227–233.
Giralt S, Estey E, Albitar M et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 1997; 89: 4531–4536.
Giralt S, Thall PF, Khouri I et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
Khouri IF, Keating M, Korbling M et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998; 16: 2817–2824.
Kishi K, Takahashi S, Gondo H et al. Second allogeneic bone marrow transplantation for post-transplant leukemia relapse: results of a survey of 66 cases in 24 Japanese institutes. Bone Marrow Transplant 1997; 19: 461–466.
Mrsic M, Horowitz MM, Atkinson K et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone Marrow Transplant 1992; 9: 269–275.
Eapen M, Giralt SA, Horowitz MM et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 2004; 34: 721–727.
Michallet M, Tanguy ML, Socie G et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de Moelle (SFGM). Br J Haematol 2000; 108: 400–407.
Russell J, Bowen T, Brown C et al. Second allogeneic transplants for leukemia using blood instead of bone marrow as a source of hematopoietic cells. Bone Marrow Transplant 1996; 18: 501–505.
Al-Qurashi F, Ayas M, Al Sharif F et al. Second allogeneic bone marrow transplantation after myeloablative conditioning analysis of 43 cases from single institution. Hematology 2004; 9: 123–129.
Wong R, Shahjahan M, Wang X et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transpl 2005; 11: 108–114.
Grandage VL, Cornish JM, Pamphilon DH et al. Second allogeneic bone marrow transplants from unrelated donors for graft failure following initial unrelated donor bone marrow transplantation. Bone Marrow Transplant 1998; 21: 687–690.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosing, C., Saliba, R., Shahjahan, M. et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone Marrow Transplant 36, 157–162 (2005). https://doi.org/10.1038/sj.bmt.1705011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705011
Keywords
This article is cited by
-
CLAG combined with total body irradiation as intensive conditioning chemotherapy prior to allogeneic hematopoietic stem cell transplantation in patients with refractory or relapsed acute myeloid leukemia
Annals of Hematology (2024)
-
Outcomes of pediatric patients who relapse after first HCT for acute leukemia or MDS
Bone Marrow Transplantation (2021)
-
Second unmanipulated allogeneic transplantation could be used as a salvage option for patients with relapsed acute leukemia post-chemotherapy plus modified donor lymphocyte infusion
Frontiers of Medicine (2021)
-
What is the role of a second allogeneic hematopoietic cell transplant in relapsed acute myeloid leukemia?
Bone Marrow Transplantation (2020)
-
Outcomes following second allogeneic stem cell transplant for disease relapse after T cell depleted transplant correlate with remission status and remission duration after the first transplant
Experimental Hematology & Oncology (2019)