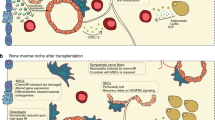
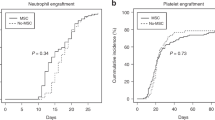
Summary:
Autologous stem cell therapy (ACT) has been proposed to prevent irradiated victims from bone marrow (BM) aplasia by grafting hematopoietic stem and progenitor cells (HSPCs) collected early after damage, provided that a functional graft of sufficient size could be produced ex vivo. To address this issue, we set up a baboon model of cell therapy in which autologous peripheral blood HSPCs collected before lethal total body irradiation were irradiated in vitro (2.5 Gy, D0 1 Gy) to mimic the cell damage, cultured in small numbers for a week in a serum-free medium in the presence of antiapoptotic cytokines and mesenchymal stem cells (MSCs) and then cografted. Our study shows that baboons cografted with expanded cells issued from 0.75 and 1 × 106/kg irradiated CD34+ cells and MSCs (n=2) exhibited a stable long-term multilineage engraftment. Hematopoietic recovery became uncertain when reducing the CD34+ cell input (0.4 × 106/kg CD34+ cells; n=3). However, platelet recovery was accelerated in all surviving cografted animals, when compared with baboons transplanted with unirradiated, unmanipulated CD34+ cells (0.5–1 × 106/kg, n=4). Baboons grafted with MSCs alone (n=3) did not recover. In all cases, the nonhematopoietic toxicity remained huge. This baboon study suggests that ACT feasibility is limited.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dainiak N . Hematologic consequences of exposure to ionizing radiations. Exp Hematol 2002; 30: 513–528.
Herodin F, Drouet M . Autologous cell therapy as a new approach to treatment of radiation-induced bone marrow aplasia: preliminary study in a baboon model. Can J Physiol Pharmacol 2002; 80: 1–7.
Bertho JM, Frick J, Demarquay C et al. Reinjection of ex vivo expanded primate bone marrow mononuclear cells strongly reduces radiation-induced aplasia. J Hematother 2002; 11: 549–564.
Bertho JM, Mathieu E, Lauby A et al. Feasibility and limits of bone marrow mononuclear cell expansion following irradiation. Int J Radiat Biol 2004; 80: 73–81.
Chute JP, Fung J, Muramoto G et al. Ex vivo culture rescues hematopoietic stem cells with long term repopulating capacity following harvest from lethally irradiated mice. Exp Hematol 2004; 32: 308–317.
Drouet M, Mathieu J, Grenier N et al. The reduction of in vitro radiation-induced-fas-related apoptosis in CD34+ progenitor cells by SCF, FLT-3 ligand, TPO, and IL-3 in combination resulted in CD34+ cell proliferation and differentiation. Stem Cells 1999; 17: 273–285.
Cronkite EP, Inoue T, Hirabayashi Y et al. Are stem cells exposed to ionizing irradiation in vivo as effective as nonirradiated transfused stem cells in restoring hematopoiesis? Exp Hematol 1993; 21: 823–825.
Norol F, Merlet P, Isnard R et al. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood 2003; 102: 4361–4368.
Bertho JM, Frick J, Demarquay C et al. Autologous cell therapy as a new treatment of accidental irradiation: study in a monkey model. Workshop Institut de Radioprotection et de Sécurité Nucléaire & Société Française de Greffe de Moelle Vaux de Cernay, France, 2003.
Chapel A, Bertho JM, Bensidhoum M et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 2003; 5: 1028–1038.
Chute JP, Saini AA, Chute DJ et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood 2002; 100: 4433–4439.
Chute JP, Muramoto G, Fung F et al. Quantitative analysis demonstrates expansion of SCID-repopulating cells and increased engraftment capacity in human cord blood following ex vivo culture with human brain endothelial cells. Stem Cells 2004; 22: 202–215.
Kadereit S, Deeds LS, Haynesworth SE et al. Expansion of LTC-ICs and maintenance of p21 and Bcl-2 expression in cord blood CD34+/CD38− early progenitors cultured over human MSCs as a feeder layer. Stem Cells 2002; 20: 573–582.
Brandt JE, Bartholomew AM, Fortman JD et al. Ex vivo expansion of autologous bone marrow CD34+ cells with porcine microvascular endothelial cells results in a graft capable of rescuing lethally irradiated baboons. Blood 1999; 94: 106–113.
Koç ON, Gerson SL, Cooper BW et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Noort WA, Kruisselbrink AB, int'Anker PS et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Exp Hematol 2002; 30: 870–878.
Angelopoulou M, Novelli E, Grove JE et al. Co-transplantation of human mesenchymal stem cells enhances myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol 2003; 31: 413–420.
Mourcin F, Grenier N, Mayol JF et al. Mesenchymal stem cells support expansion of in vitro irradiated CD34+ cells in presence of SCF, FLT-3 Ligand, TPO and IL-3: potential benefit for autologous cell therapy of irradiated victims. Radiat Res 2005; in press.
Doucet C, Ernou I, Zhang Y et al. Platelet lysates promote mesenchymal stem cell expansion: the safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 2005; in press.
Karrer EE, Lincoln JE, Hogenhout S et al. In situ isolation of mRNA from individual plant cells. Proc Natl Acad Sci USA 1995; 92: 3814–3818.
Norol F, Drouet M, Pflumio F et al. Ex vivo expansion marginally amplifies repopulating cells from baboon peripheral blood mobilized CD34+ cells. Br J Haematol 2002; 117: 924–934.
Kushida T, Inaba M, Hisha H et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune disease in MRL/lpr mice. Blood 2001; 97: 3292–3299.
Piersma AH, Ploemacher RE, Brockbank KGM . Radiation damage to femoral hemopoietic stroma measured by implant regeneration and quantitation of fibroblastic progenitors. Exp Hematol 1983; 11: 884–890.
Friedenstein AJ, Deriglasova UF, Kulagina NN et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974; 2: 83–92.
Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult mesenchymal stem cells. Science 1999; 284: 143–147.
Deans RJ, Moseley AB . Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000; 28: 875–884.
Cheng L, Qasba P, Vanguri P et al. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34+ hematopoietic progenitor cells. J Cell Physiol 2000; 184: 58–69.
Yamaguchi M, Hirayama K, Kanai M et al. Serum free coculture system for ex vivo expansion of human cord blood primitive progenitors and SCID-mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol 2001; 29: 174–182.
Devine S, Bartholomew A, Mahmud N et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001; 29: 244–255.
Devine S, Cobbs C, Jennings M et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into non-human primates. Blood 2002; 101: 2999–3001.
Javazon EH, Beggs KJ, Flake AW . Mesenchymal stem cells: paradoxes of passaging. Exp Hematol 2004; 32: 414–425.
Yahata T, Ando K, Sato T et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood 2003; 101: 2905–2913.
Mahmud N, Pang W, Cobbs C et al. Studies of the route of administration and role of conditioning with radiation on unrelated allogeneic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol 2004; 32: 494–501.
Kushida T, Inaba M, Ikebukuro K et al. A new method for bone marrow cell harvesting. Stem Cells 2000; 18: 453–456.
Drouet M, Grenier N, Delaunay C et al. Single administration of antiapoptotic cytokines soon after high dose irradiation prevents monkeys from thrombocytopenia. Blood 2004; 104: 139b (abstr.).
To LB, Haylock DN, Simmons JP et al. The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Liles WC, Broxmeyer HE, Rodger E et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003; 102: 2728–2730.
Fruehauf S . It's moving day: factors affecting peripheral blood stem cell mobilization and strategies for improvement. Br J Haematol 2003; 122: 360–375.
Brown JM, Weissman IL . Progress and prospects in hematopoietic stem cell expansion and transplantation. Exp Hematol 2004; 32: 693–695.
Herodin F, Bourin P, Mayol JF et al. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma irradiation promotes survival. Blood 2003; 101: 2609–2616.
Drouet M, Mourcin F, Grenier N et al. Single administration of stem cell factor, flt-3 ligand, megakaryocyte growth and development factor and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long term follow-up of hematopoiesis. Blood 2004; 103: 878–885.
Acknowledgements
This work was supported by a grant from the Délégation Générale pour l'Armement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drouet, M., Mourcin, F., Grenier, N. et al. Mesenchymal stem cells rescue CD34+ cells from radiation-induced apoptosis and sustain hematopoietic reconstitution after coculture and cografting in lethally irradiated baboons: is autologous stem cell therapy in nuclear accident settings hype or reality?. Bone Marrow Transplant 35, 1201–1209 (2005). https://doi.org/10.1038/sj.bmt.1704970
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704970
Keywords
This article is cited by
-
Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis
Scientific Reports (2020)
-
Use of MSCs and MSC-Educated Macrophages to Mitigate Hematopoietic Acute Radiation Syndrome
Current Stem Cell Reports (2020)
-
Mesenchymal stem/stromal cell function in modulating cell death
Stem Cell Research & Therapy (2019)
-
Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells
Leukemia (2016)
-
Sensitive Fibre-Based Thermoluminescence Detectors for High Resolution In-Vivo Dosimetry
Scientific Reports (2015)