Summary:
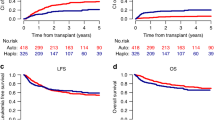
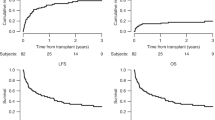
Through two consecutive trials, a policy that considered allogeneic stem cell transplantation (SCT) from a sibling donor in second rather than first complete remission (CR) in selected younger patients with acute myeloid leukemia (AML) with t(8;21)/inv(16) (core binding factor (CBF) group) or a normal karyotype (NN group) was followed by Acute Leukemia French Association (ALFA) centers. The outcome of 92 of these patients in first relapse (32 CBF, 60 NN) was reviewed with the aim of validating this strategy. The presence of an FLT3 internal tandem duplication (ITD) was retrospectively assessed in 50 patients. A total of 61 patients (66%) reached a second CR. Donor availability was an independent prognostic factor for survival in the whole patient population as well as in the CBF subset, but not in NN patients, further supporting this strategy for CBF-AMLs. In NN patients, FLT3-ITD was the main bad-prognosis factor for second CR achievement and survival, leading to consider SCT earlier, at least in FLT3-ITD patients with a donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thomas ED, Buckner CD, Clift RA et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med 1979; 310: 597–599.
Appelbaum FR, Dahlberg S, Thomas ED et al. Bone marrow transplantation or chemotherapy after remission induction for adults with acute nonlymphoblastic leukemia: a prospective comparison. Ann Intern Med 1984; 101: 581–588.
Champlin RE, Ho WG, Gale RP et al. Treatment of acute myelogenous leukemia. A prospective controlled trial of bone marrow transplantation versus consolidation chemotherapy. Ann Intern Med 1985; 102: 285–291.
Hermans J, Suciu S, Stijnen T et al. Treatment of acute myelogenous leukemia. An EBMT-EORTC retrospective analysis of chemotherapy versus allogeneic or autologous bone marrow transplantation. Eur J Cancer Oncol 1989; 25: 545–550.
Harousseau JL, Cahn JY, Pignon B et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. Blood 1997; 90: 2978–2986.
Cassileth PA, Harrington DP, Appelbaum FR et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukaemia in first remission. N Engl J Med 1998; 339: 1649–1656.
Keating S, de Witte T, Suciu S et al. The influence of HLA-matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC leukaemia cooperative group and GIMEMA. Br J Haematol 1998; 102: 1344–1353.
Burnett AK, Wheatley K, Goldstone AH et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol 2002; 118: 385–400.
Suciu S, Mandelli F, De Witte T et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMA AML-10 trial. Blood 2003; 102: 1232–1240.
Nguyen S, Leblanc T, Fenaux P et al. A white blood cell index as the main prognostic factor in t(8,21) acute myeloid leukemia (AML): a survey of 161 cases from the French AML intergroup. Blood 2002; 99: 3517–3523.
Delaunay J, Vey N, Leblanc T et al. Prognosis of inv(16)/t(16,16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML intergroup. Blood 2003; 102: 462–469.
ISCN (International System for Human Cytogenetic Nomenclature). Guidelines for Cancer Cytogenetics. In: Mitelman F (ed). Supplement to An International System for Human Cytogenetic Nomenclature. Karger: Basel, 1991, pp 1–53.
Kiyoi H, Naoe T, Nakano Y et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 1999; 93: 3074–3080.
Castaigne S, Chevret S, Archimbaud E et al. Randomized comparison of double induction and timed-sequential induction to a ‘3+7’ induction in adults with acute myeloid leukemia (AML). Long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood 2004; 104: 2467–2474.
Archimbaud E, Thomas X, Leblond V et al. Timed sequential chemotherapy for previously treated patients with acute myeloid leukemia: long-term follow-up of the etoposide, mitoxantrone, and cytarabine-86 trial. J Clin Oncol 1995; 13: 11–18.
Mayer RJ, Davis RB, Schiffer CA et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med 1994; 331: 896–903.
Kantarjian HM, Keating MJ, Walters RS et al. The characteristics and outcome of patients with late relapse acute myelogenous leukemia. J Clin Oncol 1988; 6: 232–238.
Keating MJ, Kantarjian H, Smith TL et al. Response to salvage therapy and survival after relapse in acute myelogenous leukemia. J Clin Oncol 1989; 7: 1071–1080.
Uhlman DL, Bloomfield CD, Hurd DD, Peterson BA . Prognostic factors at relapse for adults with acute myeloid leukemia. Am J Hematol 1990; 33: 110–116.
Thalhammer F, Geissler K, Jäger U et al. Duration of second complete remission in patients with acute myeloid leukemia treated with chemotherapy: a retrospective single-center study. Ann Hematol 1996; 72: 216–222.
Kern W, Schoch C, Haferlach T et al. Multivariate analysis of prognostic factors in patients with refractory and relapsed acute myeloid leukemia undergoing sequential high-dose cytosine arabinoside and mitoxantrone (S-HAM) salvage therapy: relevance of cytogenetic abnormalities. Leukemia 200; 14: 226–231.
Kottaridis PD, Gale RE, Frew ME et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Thiede C, Steudel C, Mohr B et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Schnittger S, Schoch C, Dugas M et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Döhner K, Tobis K, Ulrich R et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol 2002; 20: 3254–3261.
Preudhomme C, Sagot C, Boissel N et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association. Blood 2002; 100: 2717–2723.
van Waalwijk van Doorn-Khosrovani SB, Erpelinck C, Meijer J et al. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol J 2003; 4: 31–40.
Fröhling S, Schlenk RF, Stolze I et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol 2004; 22: 624–633.
Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, van Putten WL et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients. Blood 2003; 101: 837–845.
Baldus CD, Tanner SM, Ruppert AS et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B study. Blood 2003; 102: 1613–1618.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
de Labarthe, A., Pautas, C., Thomas, X. et al. Allogeneic stem cell transplantation in second rather than first complete remission in selected patients with good-risk acute myeloid leukemia. Bone Marrow Transplant 35, 767–773 (2005). https://doi.org/10.1038/sj.bmt.1704884
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704884
Keywords
This article is cited by
-
Different prognostic effects of core-binding factor positive AML with Korean AML registry data
Annals of Hematology (2019)
-
Importance of c-kit mutation detection method sensitivity in prognostic analyses of t(8;21)(q22;q22) acute myeloid leukemia
Leukemia (2011)
-
Interactive diagnostics in the indication to allogeneic SCT in AML
Bone Marrow Transplantation (2009)
-
Multiple Raumforderungen bei einem 30-Jährigen mit Stuhlinkontinenz
Der Internist (2009)
-
Minimal residual disease diagnostics in myeloid malignancies in the post transplant period
Bone Marrow Transplantation (2008)