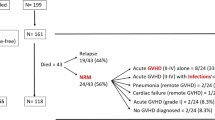
Summary:
Acute graft-versus-host disease (GVHD) increases post-transplant mortality and morbidity, but exerts a potent graft-versus-leukemia (GVL) effect. To clarify the impact of GVHD on outcome after transplant in aggressive diseases, patients with acute myeloid or lymphoblastic leukemia (AML, n=366 or ALL, n=255) in nonremission states, or chronic myelogenous leukemia (CML, n=180) in accelerated phase (AP) or blastic crisis (BC), who received allogeneic hematopoietic stem cell transplantation (HSCT) from a related donor between 1991 and 2000, were analyzed. Significant improvement in overall and disease-free survival (DFS) was detected with grade I acute GVHD in AML (P=0.0002 for overall survival and 0.0009 for DFS, respectively) and in CML (P=0.0256 and 0.0366, respectively), while the trend towards improved survival was observed in ALL. Relapse rate was lower in grade I acute GVHD than in grade II in all three diseases, suggesting that treatment for grade II GVHD may compromise the GVL effect associated with GVHD. Chronic GVHD was found to suppress relapse in CML and ALL, but not in AML, although no improvement in survival was observed in any disease category. Our results suggest that treatment for grade II acute GVHD may need to be attenuated in transplant for refractory leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Forman SJ, Schmidt GM, Nademanee AP et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. J Clin Oncol 1991; 9: 1570–1574.
Biggs JC, Horowitz MM, Gale RP et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood 1992; 80: 1090–1093.
Martin PJ, Clift RA, Fisher LD et al. HLA-identical marrow transplantation during accelerated-phase chronic myelogenous leukemia: analysis of survival and remission duration. Blood 1988; 72: 1978–1984.
Copelan EA, Grever MR, Kapoor N, Tutschka PJ . Marrow transplantation following busulfan and cyclophosphamide for chronic myelogenous leukaemia in accelerated or blastic phase. Br J Haematol 1989; 71: 487–491.
Sullivan KM, Storb R, Buckner CD et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med 1989; 320: 828–834.
Sullivan KM, Weiden PL, Storb R et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 1989; 73: 1720–1728.
Weisdorf DJ, Nesbit ME, Ramsay NK et al. Allogeneic bone marrow transplantation for acute lymphoblastic leukemia in remission: prolonged survival associated with acute graft-versus-host disease. J Clin Oncol 1987; 5: 1348–1355.
Weiden PL, Flournoy N, Thomas ED et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979; 300: 1068–1073.
Horowitz MM, Gale RP, Sondel PM et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Grebe SC, Streilein JW . Graft-versus-host reactions: a review. Adv Immunol 1976; 22: 119–221.
Korngold R, Sprent J . T cell subsets and graft-versus-host disease. Transplantation 1987; 44: 335–339.
Ferrara JL, Deeg HJ . Graft-versus-host disease. N Engl J Med 1991; 324: 667–674.
Ringden O, Hermans J, Labopin M et al. The highest leukaemia-free survival after allogeneic bone marrow transplantation is seen in patients with grade I acute graft-versus-host disease. Acute and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Leuk Lymphoma 1996; 24: 71–79.
Gratwohl A, Brand R, Apperley J et al. Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood 2002; 100: 3877–3886.
Weisdorf D, Haake R, Blazar B et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990; 75: 1024–1030.
Paulin T, Ringden O, Nilsson B et al. Variables predicting bacterial and fungal infections after allogeneic marrow engraftment. Transplantation 1987; 43: 393–398.
Miller W, Flynn P, McCullough J et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-versus-host disease. Blood 1986; 67: 1162–1167.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Gratwohl A, Hermans J, Apperley J et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood 1995; 86: 813–818.
Barrett AJ, Horowitz MM, Gale RP et al. Marrow transplantation for acute lymphoblastic leukemia: factors affecting relapse and survival. Blood 1989; 74: 862–871.
Grigg AP, Szer J, Beresford J et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukaemia. Br J Haematol 1999; 107: 409–418.
Ringden O, Sundberg B, Lonnqvist B et al. Allogeneic bone marrow transplantation for leukemia: factors of importance for long-term survival and relapse. Bone Marrow Transplant 1988; 3: 281–290.
Shiobara S, Nakao S, Ueda M et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant 2000; 26: 769–774.
Kolb HJ, Schattenberg A, Goldman JM et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood 1995; 86: 2041–2050.
Ringden O, Labopin M, Gorin NC et al. Is there a graft-versus-leukaemia effect in the absence of graft-versus-host disease in patients undergoing bone marrow transplantation for acute leukaemia? Br J Haematol 2000; 111: 1130–1137.
Locatelli F, Zecca M, Rondelli R et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood 2000; 95: 1572–1579.
Morishima Y, Kodera Y, Hirabayashi N et al. Low incidence of acute GVHD in patients transplanted with marrow from HLA-A,B,DR-compatible unrelated donors among Japanese. Bone Marrow Transplant 1995; 15: 235–239.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, I., Kami, M., Takahashi, S. et al. Clinical impact of graft-versus-host disease against leukemias not in remission at the time of allogeneic hematopoietic stem cell transplantation from related donors. The Japan Society for Hematopoietic Cell Transplantation Working Party. Bone Marrow Transplant 34, 711–719 (2004). https://doi.org/10.1038/sj.bmt.1704659
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704659
Keywords
This article is cited by
-
Improved outcome of children transplanted for high-risk leukemia by using a new strategy of cyclosporine-based GVHD prophylaxis
Bone Marrow Transplantation (2016)
-
Wiskott-Aldrich syndrome with unusual clinical features similar to juvenile myelomonocytic leukemia
International Journal of Hematology (2012)
-
Phase II study of tacrolimus and methotrexate for prophylaxis of acute graft-versus-host disease after HLA-A, B, and DRB1 genotypically mismatched unrelated bone marrow transplantation among Japanese patients
International Journal of Hematology (2009)
-
Reduced-intensity hematopoietic stem-cell transplantation for malignant lymphoma: a retrospective survey of 112 adult patients in Japan
Bone Marrow Transplantation (2005)
-
Reduced-intensity allogeneic hematopoietic stem cell transplantation for acute leukemias: ‘what is the best recipe?’
Bone Marrow Transplantation (2005)