Summary:
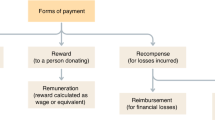
Advanced cell-based therapies are rapidly evolving and there is apparent increasing tension between regulation and introduction of new scientific advances. The model and concept of product development has introduced new challenges for laboratories developing cell-based therapies. The scale-up of experimental processes to production processes is an expensive but poorly compensated activity. The regulatory issues including personnel and facility requirements for manufacturing creates significant economic barriers to academic centers entering the field. As yet the model of product development envisioned by regulatory agencies is a patented commercial product to be manufactured by a company or its licensed manufacturers. The alternative model of advanced blood banks producing products may provide wider dissemination of products to patients. Although regulations have sometimes been perceived to be delaying advances, the regulatory agencies are evolving into a cooperative, supportive position that will ultimately speed the introduction of safe and effective cell-based therapies to broad clinical use. The vision for cell-based therapies needs scientific breadth and regulatory understanding to benefit the greatest number of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Salgaller M . A manifesto on the current state of dendritic cells in adoptive immunotherapy. Transfusion 2003; 43: 422–424.
Kaiser J . Seeking the cause of induced leukemias in X-SCID trial. Science 2003; 299: 495.
Regulations. CoF. Current good manufacturing practice for blood and blood components. Title 21(Part 606).
Anonymous. Points to consider in human somatic cell therapy and gene therapy (1991). Hum Gene Ther 1991; 2: 251–256.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gastineau, D. Will regulation be the death of cell therapy in the United States?. Bone Marrow Transplant 33, 777–780 (2004). https://doi.org/10.1038/sj.bmt.1704452
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704452