Summary:
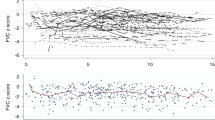
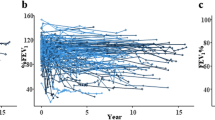
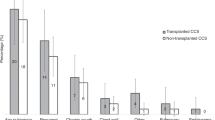
We performed serial pulmonary function tests (PFTs) consisting of spirometry and diffusing capacity in 26 children after BMT. The median follow-up was 10 years. The influence of total body irradiation (TBI) on long-term pulmonary function was of particular interest. In the 20 children who had received TBI, after an initial decrease the PFTs showed recovery, but the mean lung volumes were still significantly decreased 5 years after BMT at 10% below baseline. The proportions of children with restrictive impairment 5 and 10 years after BMT were 20 and 21%, respectively. Only one child was diagnosed with obstructive impairment. The proportions of children with isolated diffusing impairment at 5 and 10 years were 7/20 (35%) and 7/13 (54%), respectively. Six children had received chemotherapy only and showed isolated diffusing impairment as the only long-term sequela in 4/5 and 1/3 at 5 and 10 years. Our main finding was that there was little change in PFTs 1–10 years after BMT. TBI was associated with persistently decreased lung volumes in a proportion of patients, whereas chemotherapy also might have been of importance for the development of impaired gas exchange.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Soubani AO, Miller KB, Hassoun PM . Pulmonary complications of bone marrow transplantation. Chest 1996; 109: 1066–1077.
Palmas A, Tefferi A, Myers JL et al. Late-onset noninfectious pulmonary complications after allogeneic bone marrow transplantation. Br J Haematol 1998; 100: 680–687.
Nysom K, Holm K, Hesse B et al. Lung function after allogeneic bone marrow transplantation for leukaemia or lymphoma. Arch Dis Child 1996; 74: 432–436.
Kaplan EB, Wodell RA, Wilmott RW et al. Late effects of bone marrow transplantation on pulmonary function in children. Bone Marrow Transplant 1994; 14: 613–621.
Rovelli A, Pezzini C, Silvestri D et al. Cardiac and respiratory function after bone marrow transplantation in children with leukaemia. Bone Marrow Transplant 1995; 16: 571–576.
Fanfulla F, Locatelli F, Zoia MC et al. Pulmonary complications and respiratory function changes after bone marrow transplantation in children. Eur Respir J 1997; 10: 2301–2306.
Cerveri I, Fulgoni P, Giorgiani G et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120: 1900–1906.
Duncker C, Dohr D, Harsdorf S et al. Non-infectious lung complications are closely associated with chronic graft-versus-host disease: a single center study of incidence, risk factors and outcome. Bone Marrow Transplant 2000; 25: 1263–1268.
Arvidson J, Bratteby LE, Carlson K et al. Pulmonary function after autologous bone marrow transplantation in children. Bone Marrow Transplant 1994; 14: 117–123.
Gustafsson G, Kreuger A, Clausen N et al. Intensified treatment of acute childhood lymphoblastic leukaemia has improved prognosis, especially in non-high-risk patients: the Nordic experience of 2648 patients diagnosed between 1981 and 1996. Nordic Society of Paediatric Haematology and Oncology (NOPHO). Acta Paediatr 1998; 87: 1151–1161.
Lie SO, Jonmundsson G, Mellander L et al. A population-based study of 272 children with acute myeloid leukaemia treated on two consecutive protocols with different intensity: best outcome in girls, infants, and children with Down's syndrome. Nordic Society of Paediatric Haematology and Oncology (NOPHO). Br J Haematol 1996; 94: 82–88.
Lonnerholm G, Simonsson B, Arvidson J et al. Autologous bone marrow transplantation in children with acute lymphoblastic leukemia. Acta Paediatr 1992; 81: 1017–1022.
Solymar L, Aronsson PH, Bake B et al. Nitrogen single breath test, flow–volume curves and spirometry in healthy children, 7–18 years of age. Eur J Respir Dis 1980; 61: 275–286.
Hedenstrom H, Malmberg P, Agarwal K . Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir 1985; 21: 551–557.
Hedenstrom H, Malmberg P, Fridriksson HV . Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci 1986; 91: 299–310.
Nysom K, Ulrik CS, Hesse B et al. Published models and local data can bridge the gap between reference values of lung function for children and adults. Eur Respir J 1997; 10: 1591–1598.
Clark JG, Hansen JA, Hertz MI et al. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis 1993; 147: 1601–1606.
Karlberg P, Taranger J, Engstrom I et al. I. Physical growth from birth to 16 years and longitudinal outcome of the study during the same age period. Acta Paediatr Scand Suppl 1976; 258: 7–76.
Gerver WJ, De Bruin R . Relationship between height, sitting height and subischial leg length in Dutch children: presentation of normal values. Acta Paediatr 1995; 84: 532–535.
Carlson K, Backlund L, Smedmyr B et al. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994; 14: 805–811.
Tait RC, Burnett AK, Robertson AG et al. Subclinical pulmonary function defects following autologous and allogeneic bone marrow transplantation: relationship to total body irradiation and graft-versus-host disease. Int J Radiat Oncol Biol Phys 1991; 20: 1219–1227.
Quigley PM, Yeager AM, Loughlin GM . The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18: 361–367.
Schultz KR, Green GJ, Wensley D et al. Obstructive lung disease in children after allogeneic bone marrow transplantation. Blood 1994; 84: 3212–3220.
Seiden MV, Elias A, Ayash L et al. Pulmonary toxicity associated with high dose chemotherapy in the treatment of solid tumors with autologous marrow transplant: an analysis of four chemotherapy regimens. Bone Marrow Transplant 1992; 10: 57–63.
Abid SH, Malhotra V, Perry MC . Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001; 13: 242–248.
Lonnerholm G, Arvidson J, Andersson LG et al. Myocardial function after autologous bone marrow transplantation in children: a prospective long-term study. Acta Paediatr 1999; 88: 186–192.
Thomas BC, Stanhope R, Plowman PN et al. Growth following single fraction and fractionated total body irradiation for bone marrow transplantation. Eur J Pediatr 1993; 152: 888–892.
Acknowledgements
This study was supported by the Children's Cancer Foundation in Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frisk, P., Arvidson, J., Bratteby, LE. et al. Pulmonary function after autologous bone marrow transplantation in children: a long-term prospective study. Bone Marrow Transplant 33, 645–650 (2004). https://doi.org/10.1038/sj.bmt.1704393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704393
Keywords
This article is cited by
-
Chemical constituents, pharmacological activities, and uses of common ayurvedic medicinal plants: a future source of new drugs
Advances in Traditional Medicine (2023)
-
Pulmonary function after hematopoietic stem cell transplantation is significantly better in pediatric recipients following reduced toxicity compared with myeloablative conditioning
Bone Marrow Transplantation (2016)
-
High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study
Bone Marrow Transplantation (2010)
-
Bronchiolitis obliterans following pediatric allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2008)
-
Late effecten na beenmergtransplantatie
Bijblijven (2006)