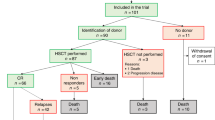
Summary:
The objective of this prospective study was to determine whether amifostine (Ethyol®) reduced conditioning-related toxicity following a regimen of busulfan (7 mg/kg) and fractionated total body irradiation (6 × 200 cGy). In all, 12 patients with advanced myelodysplastic syndrome transplanted from HLA-identical siblings were enrolled. Patients received 340 mg/m2 amifostine i.v. twice daily during conditioning (days –7 through –1). All patients developed oropharyngeal mucositis. Six patients had evidence of sinusoidal obstruction syndrome of the liver. Six patients experienced pulmonary toxicity of grades II–III. A total of 11 patients died, one with relapse and 10 with infectious complications or regimen-related toxicity. Nonrelapse causes of death included invasive aspergillosis in three, multiorgan failure in three, and idiopathic interstitial pneumonitis in two patients. One patient each died of organizing pneumonia and CMV pneumonia. One patient is alive in complete remission 31 months after transplantation. These results were not superior to those in patients conditioned with busulfan plus fractionated total body irradiation and not given amifostine, and suggest that amifostine, as administered here, has no protective effect against toxicity from this myeloablative regimen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Deeg HJ, Appelbaum FR . Hematopoietic stem cell transplantation in patients with myelodysplastic syndrome (review). Leuk Res 2000; 24: 653–663.
Anderson JE, Appelbaum FR, Schoch G et al. Allogeneic marrow transplantation for refractory anemia: a comparison of two preparative regimens and analysis of prognostic factors. Blood 1996; 87: 51–58.
Runde V, De Witte T, Arnold R et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 1998; 21: 255–261.
Deeg HJ, Storer B, Slattery JT et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood 2002; 100: 1201–1207.
Arnold R, De Witte T, van Biezen A et al. Unrelated bone marrow transplantation in patients with myelodysplastic syndromes and secondary acute myeloid leukemia: an EBMT survey. European Blood and Marrow Transplantation Group. Bone Marrow Transplant 1998; 21: 1213–1216.
Anderson JE, Appelbaum FR, Schoch G et al. Allogeneic marrow transplantation for myelodysplastic syndrome with advanced disease morphology: a phase II study of busulfan, cyclophosphamide, and total-body irradiation and analysis of prognostic factors. J Clin Oncol 1996; 14: 220–226.
Bibawi S, Abi-Said D, Fayad L et al. Thiotepa, busulfan, and cyclophosphamide as a preparative regimen for allogeneic transplantation for advanced myelodysplastic syndrome and acute myelogenous leukemia. Am J Hematol 2001; 67: 227–233.
Jurado M, Deeg HJ, Storer B et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome after conditioning with busulfan and fractionated total body irradiation is associated with low relapse rate but considerable nonrelapse mortality. Biol Blood Marrow Transplant 2002; 8: 161–169.
Capizzi RL . The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine (review). Semin Oncol 1999; 26: 3–21.
Glover D, Glick JH, Weiler C et al. WR-2721 protects against the hematologic toxicity of cyclophosphamide: a controlled phase II trial. J Clin Oncol 1986; 4: 584–588.
Kemp G, Rose P, Lurain J et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol 1996; 14: 2101–2112.
Budd GT, Ganapathi R, Adelstein DJ et al. Randomized trial of carboplatin plus amifostine versus carboplatin alone in patients with advanced solid tumors. Cancer 1997; 80: 1134–1140.
Brizel DM, Wasserman TH, Henke M et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer (erratum appears in J Clin Oncol 2000; 18(24): 4110—4111). J Clin Oncol 2000; 18: 3339–3345.
Antonadou D, Coliarakis N, Synodinou M et al. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer (erratum appears in Int J Radiat Oncol Biol Phys 2002; 52(5): 1458). Int J Radiat Oncol Biol Phys 2001; 51: 915–922.
Chauncey TR, Gooley TA, Lloid ME et al. Pilot trial of cytoprotection with amifostine given with high-dose chemotherapy and autologous peripheral blood stem cell transplantation. Am J Clin Oncol 2000; 23: 406–411.
Cronin S, Uberti JP, Ayash LJ et al. Use of amifostine as a chemoprotectant during high-dose chemotherapy in autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2000; 26: 1247–1249.
Hartmann JT, von Vangerow A, Fels LM et al. A randomized trial of amifostine in patients with high-dose VIC chemotherapy plus autologous blood stem cell transplantation. Br J Cancer 2001; 84: 313–320.
Thieblemont C, Dumontet C, Saad H et al. Amifostine reduces mucosal damage after high-dose melphalan conditioning and autologous peripheral blood progenitor cell transplantation for patients with multiple myeloma. Bone Marrow Transplant 2002; 30: 769–775.
Bennett JM, Catovsky D, Daniel MT et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982; 51: 189–199.
Greenberg P, Cox C, LeBeau MM et al. International scoring system for evaluating prognosis in myelodysplastic syndromes (published erratum appears in Blood 1998; 91: 1100). Blood 1997; 89: 2079–2088.
Storb JR, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–735.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Goerner M, Gooley T, Flowers MED et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant 2002; 8: 47–56.
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
DeLeve LD, Shulman HM, McDonald GB . Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–41.
McDonald GB, Sharma P, Matthews DE et al. Veno-occlusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
Appelbaum FR, Barrall J, Storb R et al. Bone marrow transplantation for patients with myelodysplasia. Pretreatment variables and outcome. Ann Intern Med 1990; 112: 590–597.
Bearman SI . The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005–3020.
Chapko MK, Syrjala KL, Schilter L et al. Chemoradiotherapy toxicity during bone marrow transplantation: time course and variation in pain and nausea. Bone Marrow Transplant 1989; 4: 181–186.
Deeg HJ, Spitzer TR, Cottler-Fox M et al. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant 1991; 7: 193–198.
Bornhauser M, Storer B, Slattery JT et al. Conditioning with fludarabine and targeted busulfan before transplantation of allogeneic hematopoietic stem cells. Blood 2002; 100 (Part 1): 213a (Abstr. #799).
Acknowledgements
This work was supported by the National Institutes of Health Grants CA15704, CA18029, and CA87948. MB is funded by a fellowship from the Max Kade Foundation, New York.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benesch, M., McDonald, G., Schubert, M. et al. Lack of cytoprotective effect of amifostine following HLA-identical sibling transplantation for advanced myelodysplastic syndrome (MDS): a pilot study. Bone Marrow Transplant 32, 1071–1075 (2003). https://doi.org/10.1038/sj.bmt.1704277
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704277
Keywords
This article is cited by
-
Systematic review of amifostine for the management of oral mucositis in cancer patients
Supportive Care in Cancer (2013)
-
Amifostine in the management of radiation-induced and chemo-induced mucositis
Supportive Care in Cancer (2006)