Summary:
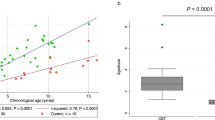
We evaluated the genotypic origin of mesenchymal stem cells (MSC) following sex-mismatched allogeneic bone marrow transplantation (BMT), and investigated the telomere dynamics in MSC in normal individuals and patients after BMT. The study population consisted of 11 patients with hematologic disorders who showed complete chimerism after BMT. Telomere length was measured in MSC using Southern blotting analysis in eight patients and 18 healthy subjects as a control group. Following culture, MSC were identified by the expression of SH2 and SH4, and lack of CD14, CD34, and CD45. All MSC showed the recipient genotype, based on the results of fluorescent in situ hybridization analysis using X-chromosome satellite probes or microsatellite DNA polymorphism analysis. The mean telomere length in MSC from normal controls was 7.2±0.53 kb (range, 6.12–7.78), and progressive telomere shortening was seen with age. There was no significant difference in MSC telomere length between the BMT group and age-matched controls. This study confirmed that the MSC isolated from the recipients of allogeneic BMT did not have the donor genotype, despite complete chimerism. Moreover, MSC were demonstrated to show progressive loss of telomere length with age, but the telomeres in MSC were not affected by BMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Majumdar MK, Thiede MA, Mosca JD et al. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998; 176: 57–66.
Deans RJ, Moseley AB . Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000; 28: 875–884.
Chamberlin W, Barone J, Kedo A, Fried W . Lack of recovery of murine hematopoietic stromal cells after irradiation-induced damage. Blood 1974; 44: 385–392.
Fried W, Chamberlin W, Kedo A, Barone J . Effects of radiation on hematopoietic stroma. Exp Hematol 1976; 4: 310–314.
Carlo-Stella C, Tabilio A, Regazzi E et al. Effect of chemotherapy for acute myelogenous leukemia on hematopoietic and fibroblast marrow progenitors. Bone Marrow Transplant 1997; 20: 465–471.
Domenech J, Roingeard F, Binet C . The mechanisms involved in the impairment of hematopoiesis after autologous bone marrow transplantation. Leukemia Lymphoma 1997; 24: 239–256.
O'Flaherty E, Sparrow R, Szer J . Bone marrow stromal function from patients after bone marrow transplantation. Bone Marrow Transplant 1995; 15: 207–212.
Galotto M, Berisso G, Delfino L et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol 1999; 27: 1460–1466.
Devine SM, Hoffman R . Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 2000; 7: 358–363.
Koc ON, Lazarus HM . Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant 2001; 27: 235–239.
Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B . Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature 1987; 328: 429–432.
Koc ON, Peters C, Aubourg P et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol 1999; 27: 1675–1681.
Awaya N, Rupert K, Bryant E, Torok-Storb B . Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol 2002; 30: 937–942.
Bianco P, Gehron Robey P . Marrow stromal stem cells. J Clin Invest 2000; 105: 1663–1668.
Horwitz EM, Prockop DJ, Fitzpatrick LA et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313.
Blackburn EH . Structure and function of telomeres. Nature 1991; 350: 569–573.
Zakian VA . Telomeres: beginning to understand the end. Science 1995; 270: 1601–1607.
Moyzis RK, Buckingham JM, Cram LS et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA 1988; 85: 6622–6626.
Blackburn EH . Telomeres: no end in sight. Cell 1994; 77: 621–623.
Vaziri H, Dragowska W, Allsopp RC et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA 1994; 91: 9857–9860.
Shay JW . Accelerated telomere shortening in bone-marrow recipients. Lancet 1998; 351: 153–154.
Wynn RF, Cross MA, Hatton C et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet 1998; 351: 178–181.
Lee J, Kook H, Chung I et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 1999; 24: 411–415.
Rufer N, Brummendorf TH, Chapuis B et al. Accelerated telomere shortening in hematological lineages is limited to the first year following stem cell transplantation. Blood 2001; 97: 575–577.
Lee JJ, Kook H, Chung IJ et al. Telomere length changes in patients with aplastic anaemia. Br J Haematol 2001; 112: 1025–1030.
Laver J, Jhanwar SC, O'Reilly RJ, Castro-Malaspina H . Host origin of the human hematopoietic microenvironment following allogeneic bone marrow transplantation. Blood 1987; 70: 1966–1968.
Agematsu K, Nakahori Y . Recipient origin of bone marrow-derived fibroblastic stromal cells during all periods following bone marrow transplantation in humans. Br J Haematol 1991; 79: 359–365.
Gordon MY . The origin of stromal cells in patients treated by bone marrow transplantation. Bone Marrow Transplant 1988; 3: 247–251.
Santucci MA, Trabetti E, Martinelli G et al. Host origin of bone marrow fibroblasts following allogeneic bone marrow transplantation for chronic myeloid leukemia. Bone Marrow Transplant 1992; 10: 255–259.
Klyushnenkova E, Shustova V, Mosca J et al. Human mesenchymal stem cells induce unresponsiveness in preactivated but not naive alloantigen-specific T cells. Exp Hematol 1999; 27: a122.
Koc ON, Gerson SL, Cooper BW et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Horwitz E, Gordon P, Koo W et al. Transplanted gene-marked marrow mesenchymal stem cells engraft and benefit children with severe osteogenesis imperfecta: a pilot trial for cell and gene therapy of mesenchymal disorders. Mol Ther 2000; 1: s279.
Koc ON, Day J, Nieder M et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215–222.
Iwama H, Ohyashiki K, Ohyashiki JH et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet 1998; 102: 397–402.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6-01GN16-0005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JJ., Nam, CE., Kook, H. et al. Constitution and telomere dynamics of bone marrow stromal cells in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant 32, 947–952 (2003). https://doi.org/10.1038/sj.bmt.1704253
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704253
Keywords
This article is cited by
-
Mixed chimerism in SCT: conflict or peaceful coexistence?
Bone Marrow Transplantation (2008)