Summary:
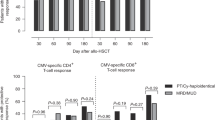
Since the incidence of cytomegalovirus (CMV) infections after hematopoietic stem cell transplantation (HSCT) may depend on the intensity of the pretreatment, we studied the incidence of CMV infections after reduced-intensity compared to myeloablative conditioning. A total of 82 patients with matched related or unrelated donors were prospectively monitored for CMV infections after HSCT by CMV-PCR techniques, CMV-antigenemia and clinical observation. A total of 45 patients received reduced-intensity conditioning consisting of fludarabine, busulfan and ATG and 37 patients received myeloablative conditioning. Leukocyte engraftment occurred after a median of 15 vs 18 days (P=0.012) and platelet engraftment after 12 days vs 20 days (P=0.001), respectively. Acute graft-versus-host disease (GVHD) grade II–IV was observed in 58 vs 54% patients (P=0.737), respectively. The onset and peak values of CMV-antigenemia and DNAemia and the incidence of CMV infections did not differ statistically significantly between the two treatment groups. Multivariate analysis confirmed CMV seropositivity of the recipient (P=0.035), acute GVHD II–IV (P=0.001) but not the type of conditioning as significant risk factors for CMV-antigenemia. In conclusion, the kinetics of CMV-antigenemia and DNAemia and the incidence of CMV infections were not statistically different in patients who received HSCT after reduced-intensity conditioning with fludarabine, busulfan and ATG compared to myeloablative conditioning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boeckh M . Current antiviral strategies for controlling cytomegalovirus in hematopoietic stem cell transplant recipients: prevention and therapy. Transplant Infect Dis 1999; 1: 165–178.
Nichols WG, Corey L, Gooley T et al. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 2002; 185: 273–282.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Slavin S, Nagler A, Naparstek E et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cyto-reduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Chakrabarti S, Mackinnon S, Chopra R et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood 2002; 99: 4357–4363.
Mohty M, Faucher C, Vey N et al. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant 2000; 26: 251–255.
Nitsche A, Steuer N, Schmidt CA et al. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin Chem 1999; 45: 1932–1937.
Schetelig J, Kroger N, Held TK et al. Allogeneic transplantation after reduced conditioning in high risk patients is complicated by a high incidence of acute and chronic graft-versus-host disease. Haematologica 2002; 87: 299–305.
Clift RA, Radich J, Appelbaum FR et al. Long-term follow-up of a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide for patients receiving allogenic marrow transplants during chronic phase of chronic myeloid leukemia. Blood 1999; 94: 3960–3962.
Przepiorka D, Khouri I, Thall P et al. Thiotepa, busulfan and cyclophosphamide as a preparative regimen for allogeneic transplantation for advanced chronic myelogenous leukemia. Bone Marrow Transplant 1999; 23: 977–981.
Schmidt CA, Oettle H, Peng R et al. Comparison of polymerase chain reaction from plasma and buffy coat with antigen detection and occurrence of immunoglobulin M for the demonstration of cytomegalovirus infection after liver transplantation. Transplantation 1995; 59: 1133–1138.
Nitsche A, Steuer N, Schmidt CA et al. Detection of human cytomegalovirus DNA by real-time quantitative PCR. J Clin Microbiol 2000; 38: 2734–2737.
Bornhauser M, Thiede C, Platzbecker U et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res 2001; 7: 2254–2262.
Nichols WG, Corey L, Gooley T et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 2001; 97: 867–874.
Junghanss C, Boeckh M, Carter RA et al. Incidence and outcome of cytomegalovirus infections following nonmyelo-ablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood 2002; 99: 1978–1985.
Faucher C, Mohty M, Chabannon C et al. Allogeneic transplantation with ATG base reduced intensity regimen and bone marrow (BM) graft is associated with rapid donor chimerism, low GVHD rate and rapid CD8+ T cells recovery. Bone Marrow Transplant 2002; 29 (Suppl 2): 157 (abstract 616).
Martino R, Caballero MD, Canals C et al. Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2001; 28: 341–347.
Bacigalupo A, Lamparelli T, Bruzzi P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Perez-Simon JA, Kottaridis PD, Martino R et al. Non-myeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood 2002; 100: 3121–3127.
Kottaridis PD, Milligan DW, Chopra R et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Acknowledgements
We would like to thank Delia Barz for excellent technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schetelig, J., Oswald, O., Steuer, N. et al. Cytomegalovirus infections in allogeneic stem cell recipients after reduced-intensity or myeloablative conditioning assessed by quantitative PCR and pp65-antigenemia. Bone Marrow Transplant 32, 695–701 (2003). https://doi.org/10.1038/sj.bmt.1704164
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704164
Keywords
This article is cited by
-
Clinical features of cytomegalovirus retinitis after solid organ transplantation versus hematopoietic stem cell transplantation
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Fludarabine-based reduced intensity conditioning transplants have a higher incidence of cytomegalovirus reactivation compared with myeloablative transplants
Bone Marrow Transplantation (2010)
-
Cytomegalovirus infection and disease after reduced intensity conditioning allogeneic stem cell transplantation: single-centre experience
Bone Marrow Transplantation (2010)
-
Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation
Bone Marrow Transplantation (2007)
-
Comparable incidence and severity of cytomegalovirus infections following T cell-depleted allogeneic stem cell transplantation preceded by reduced intensity or myeloablative conditioning
Bone Marrow Transplantation (2007)