Summary:
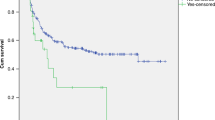
The incidence, risk factors and mortality of veno-occlusive disease (VOD) were identified for 142 pediatric hematopoietic stem cell (HSC) transplant recipients with hematological malignancies (83), solid tumors (41) and nonmalignant diseases (18). This historical cohort of 142 HSC transplant patients, from January 1993 through June 2000, was assessed by chart review. Risk factors for the development of VOD and mortality were assessed by multiple logistic regression and Kaplan–Meier survival curves respectively. The incidence of VOD was 18.3% (26/142 transplants). Multivariate analysis reconfirmed the known pretransplant risk factors of induction therapy with busulfan and transplantation with matched unrelated donor cells as significant risk factors for the development of VOD. In addition, two new risk factors, positive CMV serology in the recipient and TPN provided in the 30 days prior to transplant, were identified. Mortality in transplant patients at 100 days was greater in the VOD-positive group (10/26 (38.5%)) compared to the VOD-negative group (11/116 (9.5%) (P=0.001)). The risk of death was 4.97 times higher with 95% CIs (2.11, 11.71) for the VOD-positive group. Decreasing the risk factors for VOD may decrease mortality in this patient population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ortega JA, Donaldson SS, Ivy SP et al. Venoocclusive disease of the liver after chemotherapy with vincristine, actinomycin D, and cyclophosphamide for the treatment of rhabdomyosarcoma. Cancer 1997; 79: 2435–2439.
Ozkayniak MF, Weinberg K, Kohn D et al. Hepatic veno-occlusive disease in post-bone marrow transplantation in children conditioned with busulphan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant 1991; 7: 467–474.
Frisk P, Lonnerholm G, Oberg G . Disease of the liver following bone marrow transplantation in children: incidence, clinical course and outcome in a long-term perspective. Acta Paediatr 1998; 87: 579–583.
Locasciulli A, Testa M, Valsecchi MG et al. Morbidity and mortality due to liver disease in children undergoing allogenic bone marrow transplantation: a 10-year prospective study. Blood 1997; 90: 3799–3805.
Hasegawa S, Horibe K, Kawabe T et al. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation in children with hematologic malignancies: incidence, onset time and risk factors. Bone Marrow Transplant 1998; 22: 1191–1197.
Meresse V, Hartmann O, Vassal G et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141.
Brugieres L, Hartmann O, Benhamou E et al. Veno-occlusive disease of the liver following high-dose chemotherapy and autologous bone marrow transplantation in children with solid tumors: incidence, clinical course and outcome. Bone Marrow Transplant 1988; 3: 53–58.
Bisogno G, de Kraker J, Weirich A et al. Veno-occlusive disease of the liver in children treated for Wilms' tumor. Med Pediatr Oncol 1997; 29: 245–251.
Kullendorff CM, Bekassy AN . Hepatic veno-occlusive disease in Wilms' tumor. Eur J Pediatr Surg 1996; 6: 338–340.
Salat C, Holler E, Wolf C et al. Laboratory markers of veno-occlusive disease in the course of bone marrow and subsequent liver transplantation. Bone Marrow Transplant 1997; 19: 487–490.
McDonald GB, Sharma P, Matthews DE et al. Venoocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
DeLeve LD, Shulman HM, McDonald GB . Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–42.
Shulman HM, Hinterberger W . A review of the hepatic venoocclusive disease: liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant 1992; 10: 197–214.
Styler MJ, Crilley P, Biggs J et al. Hepatic dysfunction following busulfan and cyclophosphamide myeloablation: a retrospective, multicentre analysis. Bone Marrow Transplant 1996; 18: 171–176.
Rozman C, Carreras E, Qian C et al. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant 1996; 17: 75–80.
Kami M, Mori S, Tanikawa S et al. Risk factors for hepatic veno-occlusive disease after bone marrow transplantation: retrospective analysis of 137 cases at a single institution. Bone Marrow Transplant 1997; 20: 397–402.
Hagglund H, Remberger M, Klaesson S et al. Norethisterone treatment, a major risk factor for veno-occlusive disease in the liver after allogeneic bone marrow transplantation. Blood 1998; 92: 4568–4572.
McDonald GB, Hinds MS, Fisher LD et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Toh HC, McAfee SL, Sackstein R et al. Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant 1999; 24: 891–895.
Lee JL, Gooley T, Bensinger W et al. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors and outcome. Bone Marrow Transplant 1999; 5: 306–315.
Carreras E, Bertz H, Arcese W et al. for the European Group for Blood and Marrow Transplantation Chronic Leukemia Working Group. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European group for blood and marrow transplantation. Blood 1998; 92: 3599–3604.
Richardson P, Bearman SI . Prevention and treatment of hepatic venoocclusive disease after high-dose cytoreductive therapy. Leukemia Lymphoma 1998; 31: 267–277.
Weissman IL . Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 2000; 287: 1442–1446.
Remberger M, Ringden O . Serum levels of cytokines after bone marrow transplantation: increased IL-8 levels during severe veno-occlusive disease of the liver. Eur J Haemotol 1997; 59: 254–262.
Holler E, Kolb HJ, Kempeni J et al. Increased serum levels of tumor necrosis factor α precede major complications of bone marrow transplantation. Blood 1990; 75: 1011–1016.
Shulman HM, Fisher LB, Schoch HG et al. Venoocclusive disease of the liver after marrow transplantation: histological correlates and clinical signs and symptoms. Hepatology 1994; 19: 1171–1181.
Lemstrom KB, Aho PT, Bruggeman CA, Hayry PJ . Cytomegalovirus infection enhances mRNA expression of platelet-derived growth factor-BB and transforming growth factor-beta 1 in rat aortic allografts. Possible mechanism for cytomegalovirus-enhanced graft arteriosclerosis. Arteriosci Thromb 1994; 14: 2043–2052.
Persoons MC, Stals FS, van dam Mieras MC, Bruggeman CA . Multiple organ involvement during experimental cytomegalovirus infection is associated with disseminated vascular pathology. J Pathology 1998; 184: 103–109.
Golden MP, Hammer SM, Wanke CA, Albrecht MA . Cytomegalovirus vasculitis. Case reports and review of the literature. Medicine 1994; 73: 246–255.
Muldoon J, O'Riordan K, Rao S, Abecassis M . Ischemic colitis secondary to venous thrombosis. A rare presentation of cytomegalovirus vasculitis following renal transplantation. Transplantation 1996; 61: 1651–1653.
Yonemitsu Y, Komori K, Sueishi K, Sugimachi K . Possible role of cytomegalovirus infection in the pathogenesis of human vascular disease. Nippon Rinsho 1998; 56: 102–108.
Gibbs JP, Yang JS, Slattery JT . Comparison of human liver and small intestinal glutathione S-transferase-catalyzed busulfan conjugation in vitro. Drug Metab Dispos 1998; 26: 52–55.
Grochow LB, Jones RJ, Grundett RB et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Slattery JT, Kalorn TF, McDonald GB et al. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol 1996; 14: 1484–1494.
Brodsky R, Topolsky D, Crilley P et al. Frequency of veno-occlusive disease of the liver in bone marrow transplantation with modified busulfan/cyclophosphamide preparative regimen. Am J Clin Oncol 1990; 13: 221–225.
Essell JH, Thompson JM, Harman GS et al. Pilot trial of prophylactic ursodiol to decrease the incidence of veno-occlusive disease of the liver in allogeneic bone marrow transplant patients. Bone Marrow Transplant 1992; 10: 367–372.
Essell JH, Schroeder MT, Harman GS et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998; 128: 975–981.
Ohashi K, Tanabe J, Watanabe R et al. The Japanese multicentre open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol 2000; 64: 32–38.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barker, C., Butzner, J., Anderson, R. et al. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant 32, 79–87 (2003). https://doi.org/10.1038/sj.bmt.1704069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704069
Keywords
This article is cited by
-
Analysis of laboratory parameters before the occurrence of hepatic sinusoidal obstruction syndrome in children, adolescents, and young adults after hematopoietic stem cell transplantation
Journal of Cancer Research and Clinical Oncology (2024)
-
Prevention of veno-occlusive disease/sinusoidal obstruction syndrome: a never-ending story and no easy answer
Bone Marrow Transplantation (2023)
-
Hypofibrinolysis in pediatric patients with veno-occlusive disease in hematopoietic stem cell transplantation
Journal of Cancer Research and Clinical Oncology (2023)
-
Analysis of risk factors for hepatic sinusoidal obstruction syndrome following allogeneic hematopoietic stem cell transplantation in pediatric patients
Journal of Cancer Research and Clinical Oncology (2022)
-
Improved detection of sinusoidal obstructive syndrome using pediatric–AYA diagnostic criteria and severity grading
Bone Marrow Transplantation (2021)