Summary:
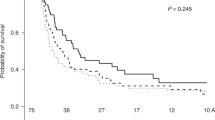
A review of the published literature, supplemented with a recent analysis of Fred Hutchinson data, has been undertaken to investigate the association of infused CD34 cell dose with various clinical outcomes after HLA-identical transplantation. Separate assessments for unrelated vs related donors and the use of bone marrow or mobilized G-PBMC have been incorporated. The three primary findings are: (1) higher CD34 dose results in better neutrophil and platelet recovery in all settings; (2) high CD34 doses (>8 × 106/kg) are associated with the development of more chronic GVHD when using related G-PBMC; (3) higher CD34 dose is correlated with improved survival after bone marrow transplantation, especially with unrelated donors. This is not seen when using G-PBMC. The data suggest that the CD34 content of the graft can have a significant impact on clinical outcome after allogeneic transplantation, but optimal dose is dependent on both donor type and stem cell source.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Storb R, Prentice RL, Thomas ED . Marrow transplantation for treatment of aplastic anemia. An analysis of factors associated with graft rejection. N Engl J Med 1977; 296: 61–66.
Wagner JE, Zahurak M, Piantadosi S et al. Bone marrow transplantation of chronic myelogenous leukemia in chronic phase: evaluation of risks and benefits. J Clin Oncol 1992; 10: 779–789.
Kernan NA, Bartsch G, Ash RC et al. Analysis of 462 transplantation from unrelated donors facilitated by the national marrow donor program. N Engl J Med 1993; 328: 593–602.
Walters M, Patience M, Leisenring W et al. Bone marrow transplantation for sickle-cell disease. N Engl J Med 1996; 336: 369–376.
Szydlo R, Goldman JM, Klein JP et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol 1997; 15: 1767–1777.
Gratwohl A, Passweg J, Baldomero H et al. For the European Group for Blood and Marrow Transplantation (EBMT). Blood and marrow transplantation activity in Europe 1997. Bone Marrow Transplant 1999; 24: 231–245.
Thomas ED, Blume KG . Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 1999; 5: 341–346.
Grigg AP, Szer J, Beresford J et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukemia. Br J Haematol 1999; 107: 409–418.
Petersdorf E, Anasetti C, Martin PJ et al. Genomics of unrelated-donor hematopoietic cell transplantation. Curr Opin Immunol 2001; 13: 582–589.
Anasetti C, Petersdorf EW, Martin PJ et al. Trends in transplantation of hematopoietic stem cells from unrelated donors. Curr Opin Hematol 2001; 8: 337–341.
Saba N, Flaig T . Bone marrow transplantation for non-malignant diseases. J Hematother Stem Cell Res 2002; 11: 377–387.
Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. Blood 2000; 95: 3702–3709.
Blaise D, Kuents M, Fortanier C et al. Randomized trial of bone marrow vs lenograstim-primed blood cell allogeneic transplantation in patients with early stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol 2000; 18: 537–546.
Bensinger W, Martin P, Storer B et al. Prospective, randomized trial of transplantation of marrow vs peripheral blood stem cells from HLA-identical siblings in patients treated for hematologic malignancies. N Engl J Med 2001; 344: 175–181.
Niederwiesser D, Pepe M, Storb R et al. Improvement in rejection, engraftment rate and survival without increase in graft-versus-host disease by high marrow cell dose in patients transplanted for aplastic anemia. Br J Haemotol 1988; 69: 23–28.
Paulin T . Importance of bone marrow cell dose in bone marrow transplantation. Clin Transplant 1992; 6: 48–54.
Sierra J, Storer B, Hansen JA et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemia burden, donor HLA-matching, and marrow cell dose. Blood 1997; 89: 4226–4235.
Russell JA, Laratt L, Brown C et al. Allogeneic blood stem cell and bone marrow transplantation for acute myelogenous leukemia and myelodysplasia: influence of stem cell source on outcome. Bone Marrow Transplant 1999; 24: 1177–1183.
Barrett AJ, Ringden O, Zhang MJ et al. Effect of nucleated marrow cell dose on relapse and survival in identical twin bone marrow transplants for leukemia. Blood 2000; 95: 3323–3327.
Dominietto A, Raiola AM, Van Lint MT et al. Factors influencing hematologic recovery after allogeneic stem cells transplants (HSCT): graft versus host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol 2001; 112: 219–227.
Rocha V, Carmagnat MV, Chevret S et al. Influence of bone marrow graft lymphocyte subsets on outcome after HLA- identical sibling transplants. Exp Hematol 2001; 29: 1347–1352.
Mavroudis D, Read E, Cottler-Fox M et al. CD34+ cell dose predicts survival, post-transplant morbidity, and the rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood 1996; 88: 3223–3229.
Anasetti C, Heimfeld S, Rowley S et al. Higher CD34 cell dose is associated with improved survival after marrow transplantation from unrelated donors. Blood 1999; 94: 561a.
Przepiorka D, Smith T, Folloder J et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 1999; 94: 1465–1470.
Ilhan O, Arsian O, Arat M et al. The impact of CD34+ cell dose on engraftment in allogeneic peripheral blood stem cell transplantation. Transfusion Sci 1999; 20: 69–71.
Davies SM, Kollman C, Anasetti C et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the National Marrow Donor Program. Blood 2000; 96: 4096–4102.
Cornelissen JJ, Carston M, Kollman C et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus leukemia effect and risk factors determining outcome. Blood 2001; 97: 1572–1577.
Morariu-Zamfir R, Rocha V, Devergie A et al. Influence of CD34+ marrow cell dose on outcome of HLA-identical sibling allogeneic bone marrow transplants in patients with chronic myeloid leukemia. Bone Marrow Transplant 2001; 27: 575–580.
Zaucha JM, Gooley T, Bensinger WI et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood 2001; 98: 3221–3227.
Bittencourt H, Rocha V, Chevret S et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood 2002; 99: 2726–2733.
Gluckman E . Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol 2000; 28: 1197–1205.
Locatelli F, Rocha V, Chastang C et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Eurocord-Cord Blood Transplant Group. Blood 1999; 93: 3662–3671.
Wagner JE, Barker JN, DeFor TE et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002; 100: 1611–1618.
Urbano-Ispizua A, Rozman C, Pimental P et al. The number of CD3+ cells is the most important factor for graft failure after allogeneic transplantation of CD34+ selected cells from peripheral blood from HLA-identical siblings. Blood 2001; 97: 383–387.
Urbano-Ispizua A, Carreras E, Marin P et al. Allogeneic transplantation of CD34+ selected cells from peripheral blood from HLA-identical siblings: detrimental effect of a high number of donor CD34+ cells. Blood 2001; 98: 2352–2357.
Keever-Taylor CA, Klein JP, Eastwood D et al. Factors affecting neutrophil and platelet reconstitution after T cell-depleted bone marrow transplantation: differential effects of growth factor type and role of CD34+ cell dose. Bone Marrow Transplant 2001; 27: 791–800.
Passweg JR, Meyer-Monard S, Gregor M et al. High stem cell dose will not compensate for T cell depletion in allogeneic non-myeloablative stem cell transplantation. Bone Marrow Transplant 2002; 30: 267–271.
Sutherland DR, Anderson L, Keeney M et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother 1996; 5: 213–226.
Johnsen HE, Knudsen LM . Nordic flow cytometry standards for CD34+ cell enumeration in blood and leukapheresis products: report from the Second Nordic Workshop. J Hematother 1996; 5: 237–245.
Chang A, Ma DDF . The influence of flow cytometric gating strategy on the standardization of CD34+ cell quantitation: an Australian multicenter study. J Hematother 1996; 5: 605–616.
Gratama JW, Orfao A, Barnett D et al. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells. European Working Group on Clinical Cell Analysis. Cytometry 1998; 34: 128–142.
Leuner S, Arland M, Kahl C et al. Enumeration of CD34-positive hematopoietic progenitor cells by flow cytometry: comparison of a volumetric assay and the ISHAGE gating strategy. Bone Marrow Transplant 1998; 22: 699–706.
Brando B, Barnett D, Janossy G et al. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry 2000; 42: 327–346.
Serke S, Johnsen HE . A European reference protocol for quality assessment and clinical validation of autologous hematopoietic blood progenitor and stem cell grafts. Bone Marrow Transplant 2001; 27: 463–470.
Leisenring JYW, Fritschle W, Heimfeld S et al. Enumeration of HPC in mobilized peripheral blood with the sysmex SE9500 predicts final CD34+ cell yield in the apheresis collection. Bone Marrow Transplant 2000; 25: 1157–1164.
Graf L, Heimfeld S, Torok-Storb B . Comparison of gene expression in CD34+ cells from bone marrow and GCSF-mobilized peripheral blood by high-density oligonucleotide array analysis. Biol Blood Marrow Transplant 2001; 7: 486–494.
Demirer T, Ihlan O, Arat M et al. CD41+ and CD42+ hematopoietic progenitor cells may predict platelet engraftment after allogeneic peripheral blood stem cell transplantation. J Clin Apher 2001; 16: 67–43.
Pocock C, Szydlo R, David J et al. Stem cell transplantation for chronic myeloid leukemia: the role of infused marrow cell dose. Hematology J 2001; 2: 265–271.
Castro-Malaspina H, Harris RE, Gajewski J et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood 2002; 99: 1943–1951.
Rocha V, Myriam L, Gluckman E et al. Relevance of bone marrow cell dose on allogeneic transplantation outcomes for patients with acute myeloid leukemia in first complete remission: results of a European Survey. J Clin Oncol 2002; 20: 4324–4330.
Singhal S, Powles R, Treleaven J et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2 × 106 CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant 2000; 26: 489–496.
Waller EK, Rosenthal H, Jones TW et al. Larger numbers of CD4-bright dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood 2001; 97: 2948–2956.
Arpinati M, Green CL, Heimfeld S et al. G-CSF mobilizes T helper 2-inducing dendritic cells. Blood 2000; 95: 2484–2490.
Acknowledgements
This work was supported by NIH Grants HL66947, CA15704, CA18029, DK56465, and HL54881.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heimfeld, S. HLA-identical stem cell transplantation: is there an optimal CD34 cell dose?. Bone Marrow Transplant 31, 839–845 (2003). https://doi.org/10.1038/sj.bmt.1704019
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704019
Keywords
This article is cited by
-
Salvage treatment with plerixafor in poor mobilizing allogeneic stem cell donors: results of a prospective phase II-trial
Bone Marrow Transplantation (2021)
-
Is it time to revisit our current hematopoietic progenitor cell quantification methods in the clinic?
Bone Marrow Transplantation (2012)
-
Higher CD3+ and CD34+ cell doses in the graft increase the incidence of acute GVHD in children receiving BMT for thalassemia
Bone Marrow Transplantation (2012)
-
Graft source determines human hematopoietic progenitor distribution pattern within the CD34+ compartment
Bone Marrow Transplantation (2011)
-
Allo-SCT using reduced-intensity conditioning against advanced pancreatic cancer: a Japanese survey
Bone Marrow Transplantation (2008)