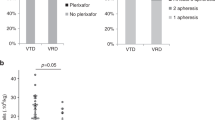
Summary:
In total, 18 of 26 double high-dose chemotherapies (HDCT) in pediatric solid tumors were rescued with peripheral blood stem cells collected during a single leukapheresis round (single-harvest group, SHG). In the remaining eight HDCT, additional leukapheresis were necessary after the first HDCT (HDCT1) to rescue the second HDCT (HDCT2) (double-harvest group, DHG). Stem cell collection after HDCT1 was inefficient and delayed in patients who had received prior chemotherapy before HDCT1. The interval between HDCT1 and HDCT2 was shorter in SHG than in DHG (median 62.5 days vs 178.5 days, P-value=0.002). Hematologic recovery in HDCT2 was delayed compared to HDCT1. However, there was no difference in hematologic recovery between SHG and DHG. A high rate of treatment-related mortality (TRM) was recorded during HDCT2, but there was no evidence that the shorter interval caused a higher rate of TRM (P-value=0.454). The probability of disease-free survival at 2 years after HDCT2 in the SHG and DHG were 66.7 and 25.0%, respectively (P-value=0.031). Therefore, to administer the second HDCT earlier in double HDCT, and thus to improve the survival of patients with high-risk solid tumors, the single-harvest approach is recommended rather than the double-harvest approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kanami NR . Autotransplants for neuroblastoma. Bone Marrow Transplant 1996; 17: 301–304.
Heideman RL . Overview of treatment of infant central nervous system tumors: medulloblastoma as a model. J Pediatr Hematol Oncol 2001; 23: 268–271.
Warkentin PI, Brochstein JA, Standjord SE et al. High dose chemotherapy followed by autologous stem cell rescue for recurrent Wilms' tumor. J Clin Oncol 1993; 12: 414 (abstract).
Fitoussi O, Simon D, Brice P et al. Tandem transplant of peripheral blood stem cells for patients with poor-prognosis Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant 1999; 24: 747–755.
Ayash LJ, Elias A, Schwarz G et al. Double dose-intensive chemotherapy with autologous stem-cell support for metastatic breast cancer: no improvement in progression-free survival by the sequence of high dose melphalan followed by cyclophosphamide, thiotepa, and carboplatin. J Clin Oncol 1996; 14: 2984–2992.
Philip T, Ladenstein R, Zucker JM et al. Double megatherapy and autologous bone marrow transplantation for advanced neuroblastoma: the LMCE2 study. Br J Cancer 1993; 67: 119–127.
Kawa-Ha K, Yumura-Yagi K, Inoue M et al. Results of single and double autografts for high risk neuroblastoma patients. Bone Marrow Transplant 1996; 17: 957–962.
Valteau-Couanet D, Rubie H, Meresse V et al. Phase I–II study of interleukin-2 after high-dose chemotherapy and autologous bone marrow transplantation in poorly responding neuroblastoma. Bone Marrow Transplant 1995; 16: 515–520.
Pession A, Prete A, Locatelli F et al. Immunotherapy with low-dose recombinant interleukin-2 after high dose chemotherapy and autologous stem cell transplantation in neuroblastoma. Br J Cancer 1998; 78: 528–533.
Matthay KK, Villablanca JG, Seeger RC et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med 1999; 341: 1165–1173.
Matthay KK, Atkinson J, Stram D et al. Patterns of relapse after autologous purged bone marrow transplanation for neuroblastoma: A Children's Cancer Group Pilot Study. J Clin Oncol 1993; 11: 2226–2233.
Garaventa A, Rondelli R, Lanino E et al. Myeloablative therapy and bone marrow rescue in advanced neuroblastoma. Report from the Italian Bone Marrow Transplant Registry. Bone Marrow Transplant 1996; 18: 125–130.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sung, K., Yoo, K., Chung, E. et al. Successive double high-dose chemotherapy with peripheral blood stem cell rescue collected during a single leukapheresis round in patients with high-risk pediatric solid tumors: a pilot study in a single center. Bone Marrow Transplant 31, 447–452 (2003). https://doi.org/10.1038/sj.bmt.1703869
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703869