Abstract
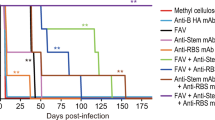
The use of zanamivir in seven patients with influenza (three A and four B) post allograft is described. Inhaled zanamivir (10 mg twice daily) was continued from the diagnosis of influenza until excretion of virus ceased (median duration 15 days, range 5 to 44 days). There was no toxicity attributable to zanamivir and rapid resolution of influenza symptoms was seen. There was no mortality due to influenza in the seven patients. The good outcome of 30 previous patients with influenza post transplant is described. A randomised multicentre study would be required to demonstrate efficacy.
Bone Marrow Transplantation (2002) 29, 113–115. doi:10.1038/sj.bmt.1703343
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Marks DI, Cullis JO, Ward KN et al. Allogeneic bone marrow transplantation for chronic myeloid leukaemia using sibling and volunteer unrelated donors Ann Intern Med 1993 119: 207 214
Williamson ECM, Millar MR, Steward CG et al. Infections in adults undergoing unrelated donor bone marrow transplantation Br J Haematol 1999 104: 560 568
Hayden FG, Osterhaus ADME, Treanor JJ et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections New Engl J Med 1997 337: 874 880
Anonymous. Randomised trial of efficacy and safety of inhaled zanamavir in treatment of influenza A and B virus infections. The MIST Study Group Lancet 1999 352: 668 669
Hayden FG, Gubareva LV, Monto AS et al. Inhaled zanamavir for the prevention of influenza in families. Zanamavir Family Study Group New Engl J Med 2000 343: 1331 1332
McCarthy AJ, Kingman HM, Kelly C et al. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation Bone Marrow Transplant 1999 24: 1315 1322
Bowden RA . Other viruses after hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ (eds) Hematopoietic Cell Transplantation Blackwell Science, MA 1999 pp 618 626
Williamson JC, Pegram PS . Respiratory distress associated with zanamavir New Engl J Med 2000 342: 661 662
Gubareva LV, Metrosovich MN, Brenner MK et al. Evidence for zanamavir resistance in an immunocompromised child infected with influenza B virus J Infect Dis 1998 178: 1257 1262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johny, A., Clark, A., Price, N. et al. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant 29, 113–115 (2002). https://doi.org/10.1038/sj.bmt.1703343
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703343
Keywords
This article is cited by
-
Serum IgM levels independently predict immune response to influenza vaccine in long-term survivors vaccinated at >1 year after undergoing allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2017)
-
Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation
Journal of Hematology & Oncology (2013)
-
The clinical features and outcome of 2009 H1N1 influenza infection in allo-SCT patients: a British Society of Blood and Marrow Transplantation study
Bone Marrow Transplantation (2012)