Abstract
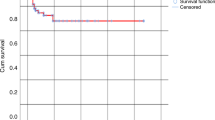
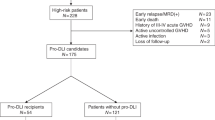
T cell depletion of the graft increases graft failure and relapse rate in allogeneic PBSC transplantation. Delayed lymphocyte add-back after T cell-depleted transplants might prevent these complications. We present 22 consecutive allogeneic PBSC transplants from related histocompatible donors with positive selection of CD34+ cells. Recipients received prophylactic donor lymphocyte infusions (DLI) depending on their risk of relapse and of developing GVHD. Patients were considered at high risk of relapse with AML > first CR, ALL > second CR, and CML in accelerated or blastic phase. Patients were considered at high risk of developing GVHD if older than 35 years, or with a donor sensitized through previous pregnancy or blood transfusion. Patients at high risk of relapse and low risk of GVHD were scheduled to receive three DLI. Patients at low risk of relapse and high risk of GVHD did not receive DLI. The remaining patients were scheduled to receive two DLI. The DLI were administered on days +28 (2 × 105/kg), +60 (2 × 105/kg) and +90 (2 × 106/kg) after transplant. G-CSF mobilized peripheral stem cells from healthy donors were positively selected by an immunomagnetic method. The mean CD34+ cells and CD3+ cells infused were 4.4 × 106(range 1.9–10.6) and 0.085 × 105 (range 0.01–0.67). Cyclosporin A was given to prevent GVHD. All the patients engrafted. Twenty-two prophylactic DLI were performed in 12 patients: seven developed acute GVHD (one case grade III–IV) and none presented pancytopenia. At a mean follow-up of 585 days (range 89–1103), 14 patients were alive in CR, one patient was alive in relapse, four patients had died of relapse and three had died of transplant-related complication. Individually adjusted prophylactic DLI at the doses we used with an escalating schedule allowed an acceptable GVHD rate and a good engraftment of donor hematopoiesis. Bone Marrow Transplantation (2001) 28, 963–968.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marmont AM, Horowitz MM, Gale RP et al. T cell depletion of HLA-identical transplants in leukemia Blood 1991 78: 2120–2130
Urbano-Ispizua A, Rozman C, Pimentel P et al. The number of donor CD3(+) cells is the most important factor for graft failure after allogeneic transplantation of CD34(+) selected cells from peripheral blood from HLA-identical siblings Blood 2001 97: 383–387
Goldman JM, Gale RP, Horowitz MM et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase: increased risk of relapse associated with T cell depletion Ann Intern Med 1998 108: 806–814
Gallardo D, García-López J, Sureda A et al. Low dose donor CD8+ in the CD4-depleted graft prevent allogeneic marrow graft rejection and severe graft-versus-host disease for chronic myeloid leukemia patients in first chronic phase Bone Marrow Transplant 1997 20: 945–952
Verdonck LF, Dekker AW, de Gast GC et al. Allogeneic bone marrow transplantation with a fixed low number of T cells in the graft Blood 1994 83: 3090–3096
Bahçeci E, Read EJ, Leitman S et al. CD34+ cell dose predicts relapse and survival after T cell depleted HLA-identical haematopoietic stem cell transplantation (HSCT) for haematological malignancies Br J Haematol 2000 108: 408–414
Naparsteck E, Or R, Nagler A et al. T cell-depleted allogeneic bone marrow transplantation for acute leukaemia using Campath-1 antibodies and post-transplant administration of donor's peripheral blood lymphocytes for prevention of relapse Br J Haematol 1995 89: 506–515
Martino R, Martín-Henao G, Sureda A et al. Allogeneic peripheral blood stem cell transplantation with CD34+-cell selection and delayed T cell add-back in adults. Results of a single center pilot study Haematologica 2000 85: 1165–1171
Lee CK, Gingrich RD, deMagalhaes-Silverman M et al. Prophylactic reinfusion of T cells for T cell-depleted allogeneic bone marrow transplantation Biol Blood Marrow Transplant 1999 5: 15–27
Kolb HJ, Schattenberg A, Goldman JM et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients Blood 1995 86: 2041–2025
Lokhorst HM, Schattenberg A, Cornelissen JJ et al. Donor lymphocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation Blood 1997 90: 4206–4211
Collins RH, Shpilberg O, Drobyski WR et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation J Clin Oncol 1997 15: 433–444
Carlens S, Remberger M, Aschan J, Ringdén O . The role of disease stage in the response to donor lymphocyte infusions as treatment of leukemia relapse Biol Blood Marrow Transplant 2001 7: 31–38
Johnson BD, Truitt RL . Delayed infusion of immunocompetent donor cells after bone marrow transplantation breaks graft-host tolerance and allows for persistent antileukemic reactivity without severe graft-versus-host disease Blood 1995 85: 3302–3312
Bensinger WI, Clift R, Martin P et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation Blood 1996 88: 2794–3800
Solano C, Martínez C, Brunet S et al. Chronic graft-versus-host disease after allogeneic peripheral blood progenitor cell or bone marrow transplantation from matched related donor. A case-control study. Spanish Group of allo-PBT Bone Marrow Transplant 1998 22: 1129–1135
Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogenic transplantation Blood 2000 95: 3702–3709
Urbano-Ispizua A, Solano C, Brunet S et al. Allogeneic transplantation of selected CD34+ cells from peripheral blood: experience of 62 cases using immunoadsorption or immunomagnetic technique. Spanish Group of Allo-PBT Bone Marrow Transplant 1998 22: 519–525
Cornelissen JJ, Fibbe WE, Schattenberg AVMB et al. A retrospective Dutch study comparing T cell-depleted allogeneic blood stem cell transplantation vs T cell-depleted allogeneic bone marrow transplantation Bone Marrow Transplant 1998 21: (Suppl. 3) S66–S70
Knauf W, Fietz T, Schrezenmeier H, Thiel E . CD34-selected alloPBSCT and adoptive immunotherapy Bone Marrow Transplant 2000 25: S2–S5
Martín-Henao GA, Picón M, Amill B et al. Isolation of CD34+ progenitor cells from peripheral blood by use of an automated immunomagnetic selection system: factors affecting the results Transplantation 2000 40: 35–44
Kaplan EL, Meier P . Non-parametric estimation from incomplete observations J Am Stat Assoc 1958 53: 457–481
Kernan NA, Collins NH, Juliano L et al. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-versus-host disease Blood 1986 68: 770–773
Sierra J, Storer B, Hansen JA et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose Blood 1997 89: 4226–4235
Mavroudis D, Read E, Cottler-Fox M et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies Bone Marrow Transplant 1996 88: 3223–3229
Mackinnon S, Papadopoulos EB, Carabasi MH et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease Blood 1995 86: 1261–1268
Bacigalupo A, Soracco M, Vassallo F et al. Donor lymphocyte infusions (DLI) in patients with chronic myeloid leukemia following allogeneic bone marrow transplantation Bone Marrow Transplant 1997 19: 927–937
Johnson BD, Drobyski WR, Truitt RL . Delayed infusion of normal donor cells after MHC-matched bone marrow transplantation provides an antileukemia reaction without graft-versus-disease Bone Marrow Transplant 1993 11: 329–336
Baron F, Siquet J, Baudoux E et al. Pre-emptive CD8-depleted donor lymphocytes infusions (DLI) after CD34-selected allogeneic peripheral blood stem-cell (PBSC) transplantation Bone Marrow Transplant 2001 27: (Suppl. 1): OS240
Barret AJ, Mavroudis D, Tisdale J et al. T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect Bone Marrow Transplant 1998 21: 543–551
Weiss L, Reich S, Slavin S . Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders: treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL-2 activated cell-mediated immunotherapy Cancer Invest 1992 10: 19–26
Acknowledgements
This work was supported by the Fundación Internacional José Carreras para la Lucha contra la Leucemia FIJC/AG00. MRL is a recipient of a grant BEFI 99/9136 from the Spanish Health Ministry.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrá, C., Rodríguez-Luaces, M., Gallardo, D. et al. Individually adjusted prophylactic donor lymphocyte infusions after CD34-selected allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 28, 963–968 (2001). https://doi.org/10.1038/sj.bmt.1703277
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703277
Keywords
This article is cited by
-
The graft-versus-leukemia effect of prophylactic donor lymphocyte infusions after allogeneic stem cell transplantation is equally effective in relapse prevention but safer compared to spontaneous graft-versus-host disease
Annals of Hematology (2023)
-
Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT
Bone Marrow Transplantation (2016)
-
T Cell Immunotherapy for Immune Reconstitution and GVHD Prevention After Allogeneic Hematopoietic Stem Cell Transplantation
Current Stem Cell Reports (2015)
-
Treatment options for the management of acute leukaemia relapsing following an allogeneic transplant
Bone Marrow Transplantation (2008)
-
Donor lymphocyte infusions: the long and winding road: how should it be traveled?
Bone Marrow Transplantation (2008)