Abstract
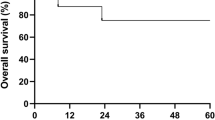
Severe aplastic anemia (SAA) is well described in children following liver transplantation for fulminant hepatic failure (FHF) secondary to non-A, non-B, non-C hepatitis, and is associated with a high mortality rate. Successful immunosuppressive treatment of SAA following liver transplantation has been reported, but death from infectious complications is not uncommon. We report the 8-year follow-up of a 3.5-year-old boy who underwent successful HLA-identical sibling donor bone marrow transplant for SAA 7 months following orthotopic liver transplant for non-A, non-B, non-C hepatitis. His post-bone marrow transplantation course was uneventful with no evidence of liver toxicity. Eight months following BMT he developed renal cell carcinoma metastatic to lymph nodes which was treated surgically. Six years following BMT he developed a mucoepidermoid carcinoma of the parotid gland also treated surgically. Despite these malignancies, he is currently well 8 years following liver and bone marrow transplantation, without signs of GVHD, growth failure or liver graft rejection. This is the first report of long-term follow-up of bone marrow transplantation for SAA following liver transplantation. The occurrence of two subsequent malignancies in this child underscores the need for close follow-up of future similar cases. Bone Marrow Transplantation (2001) 28, 523–526.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cattral MS, Langnas AN, Markin RS et al. Aplastic anemia after liver transplantation for fulminant liver failure Hepatology 1994 20: 813–818
Tzakis AG, Arditi M, Whitington PF et al. Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis New Engl J Med 1988 319: 393–396
Langnas AN, Markin RS, Cattral MS et al. Parvovirus B19 as a possible causative agent of fulminant liver failure and associated aplastic anemia Hepatology 1995 22: 1661–1665
Sato S, Fuchinoue S, Abe M et al. Successful cytokine treatment of aplastic anemia following living-related orthotopic liver transplantation for non-A, non-B, non-C hepatitis Clin Transplant 1999 13: 68–71
Rebuffoni D . Benzene can cause serious ailments Minneapolis Star & Tribune July 1 1992
Kawahara K, Storb R, Sanders J, Petersen FB . Successful allogeneic bone marrow transplantation in a 6.5-year-old male for severe aplastic anemia complicating orthotopic liver transplantation for fulminant non-A-non-B hepatitis Blood 1991 78: 1140–1143
Hagglund H, Winiarski J, Ringden O et al. Successful allogeneic bone marrow transplantation in a 2.5-year-old boy with ongoing cytomegalovirus viremia and severe aplastic anemia after orthotopic liver transplantation for non-A, non-B, non-C hepatitis Transplant 1997 64: 1207–1208
Trede NS, Warwick AB, Rosoff PM et al. Tacrolimus (FK506) in allogeneic bone marrow transplantation for severe aplastic anemia following orthotopic liver transplantation Bone Marrow Transplant 1997 20: 257–260
Socie G, Curtis RE, Dee HJ et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia J Clin Oncol 2000 18: 348–357
Lowsky R, Lipton J, Fyles G et al. Secondary malignancies after bone marrow transplantation in adults J Clin Oncol 1994 12: 2187–2192
Bhatia S, Ramsay NK, Steinbuch M et al. Malignant neoplasms following bone marrow transplantation Blood 1996 87: 3633–3639
Deeg HJ, Socie G, Schoch G et al. Malignancies after marrow transplantation for aplastic anemia and Fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients Blood 1996 87: 386–392
Pierga JY, Socie G, Gluckman E et al. Secondary solid malignant tumors ocurring after bone marrow transplantation for severe aplastic anemia given thoraco-abdominal irradiation Radiother Oncol 1994 30: 55–58
Sheiner PA, Magliocca JF, Bodian CA et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant Transplant 2000 69: 781–789
Offner G, Latta K, Hoyer PF et al. Kidney transplanted children come of age Kidney Int 1999 55: 1509–1517
Kishikawa H, Ichikawa Y, Yazawa K et al. Malignant neoplasm in kidney transplantation Int J Urol 1998 5: 521–525
Niedobitek G, Mutimer DJ, Williams A et al. Epstein–Barr virus infection and malignant lymphomas in liver transplant recipients Int J Can 1997 73: 514–520
Bakr MA, Sobh M, el-Agroudy A et al. Study of malignancy among Egyptian kidney transplant recipients Transplant Proc 1997 29: 3067–3070
Montagnino G, Lorca E, Tarantino A et al. Cancer incidence in 854 kidney transplant recipients from a single institution: comparison with normal population and with patients under dialytic treatment Clin Transplant 1996 10: 461–469
Tan-Shalaby J, Tempero M . Malignancies after liver transplantation: a comparative review Semin Liver Dis 1995 15: 156–154
Sheil AG . Malignancy following liver transplantation: a report from the Australian Combined Liver Transplant Registry Transplant Proc 1995 27: 1247
Katai M, Sakurai A, Ichikawa K et al. Sarcomatoid renal cell carcinoma with widespread metastases to liver and bones in a kidney transplant recipient Transplant 1997 63: 1361–1363
Snyder R . Recent developments in the understanding of benzene toxicity and leukemogenesis Drug Chem Toxicol 2000 23: 13–25
Hicks J, Flaitz C . Mucoepidermoid carcinoma of salivary glands in children and adolescents: assessment of proliferation markers Oral Oncol 2000 36: 454–460
Loy TS, McLaughlin R, Odom LF et al. Mucoepidermoid carcinoma of the parotid as a second malignant neoplasm in children Cancer 1989 64: 2174–2177
Eapen M, Ramsay NKC, Robison LL et al. Late outcomes after bone marrow transplantation for severe aplastic anemia Br J Haematol 2000 111: 754–760
Storb R, Blume KG, O'Donnell MR et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantation: the experience in four centers Biol Blood Marrow Transplant 2001 7: 39–44
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perkins, J., Neglia, J., Ramsay, N. et al. Successful bone marrow transplantation for severe aplastic anemia following orthotopic liver transplantation: long-term follow-up and outcome. Bone Marrow Transplant 28, 523–526 (2001). https://doi.org/10.1038/sj.bmt.1703177
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703177
Keywords
This article is cited by
-
Acute graft versus host disease after orthotopic liver transplantation
Journal of Hematology & Oncology (2012)
-
Tolerance to liver allograft after allogeneic hematopoietic cell transplantation for severe aplastic anemia from the same HLA-matched sibling donor
Bone Marrow Transplantation (2012)
-
Hematopoietic stem-cell transplantation following solid-organ transplantation in children
Bone Marrow Transplantation (2011)
-
Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient
Bone Marrow Transplantation (2004)
-
Should we be performing more combined hematopoietic stem cell plus solid organ transplants?
Bone Marrow Transplantation (2003)