Abstract
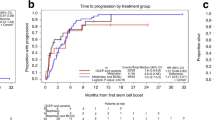
Two cycles of high-dose chemotherapy with stem cell support (HDC) may increase the total dose delivered and dose intensity. A brief induction phase and different non-cross-resistant agents for each HDC cycle were used to avoid drug resistance. Twenty-six women with metastatic BC had induction and stem cell mobilization with two cycles of doxorubicin/G-CSF given every 14 days. Patients with stable disease or better after induction received HD CTCb followed by HD melphalan and dose-escalated paclitaxel. At 475 mg/m2 of paclitaxel by 24-h infusion, dose-limiting transient peripheral sensory neuropathy was encountered. No toxic deaths occurred. Complete and near complete response after completion of therapy was achieved in 22 (85%) of 26 patients. The median EFS was 38 months. The median OS has not yet been reached. At a median follow-up of 33 (25–43) months, actuarial EFS and OS were 54% (95% confidence interval (CI), 39–69%) and 69% (95% CI, 56–79%), respectively. This double transplant approach lasts only 14 weeks and is feasible, safe, and tolerable. Whilst selection biases may in part contribute to favorable EFS and OS, a randomized comparison of standard therapy vs double transplant in both metastatic and locally advanced breast cancer is warranted. Bone Marrow Transplantation (2001) 28, 447–454.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sledge GW, Neuberg D, Ingle J et al. Phase III trial of doxorubicin versus paclitaxel versus doxorubicin plus paclitaxel as first-line therapy for metastatic breast cancer: an intergroup trial Proc ASCO 1997 16: 1a (A-2)
Antman KH, Rowlings PA, Vaughan WP et al. High-dose chemotherapy with autologous hematopoietic stem-cell support for breast cancer in North America J Clin Oncol 1997 15: 1870–1879
Ayash LJ, Wheeler C, Fairclough D et al. Prognostic factors for prolonged progression-free survival with high-dose chemotherapy with autologous stem cell support for advanced breast cancer J Clin Oncol 1995 13: 2043–2049
Holden S, Teicher B, Ayash L, Frei E III . A preclinical model for sequential high dose chemotherapy Cancer Chemother Pharmacol 1995 36: 61–64
Elias AD, Wheeler C, Richardson P et al. A phase I trial of double transplant for metastatic breast cancer: sequence and interval considerations Proc Am Soc for Blood and Marrow Transplantation Annual Meeting 1998 Carden Jennings: Charlottesville, VA, USA (Abstr.)
Frei E III, Richardson P, Ara G et al. Tandem high-dose chemotherapy with stem cell rescue (HD-SCR) in patients with breast cancer – effect of sequence Cancer Chemother Pharmacol 2000 45: 239–246
Frei E III, Richardson P, Avigan D et al. The interval between courses of high-dose chemotherapy with stem cell rescue: therapeutic hypotheses Bone Marrow Transplant 1999 24: 939–945
Teicher BA, Holden SA, Herman TS et al. Characteristics of five human tumor cell lines and sublines resistant to cis-diamminedichloroplatinum (II) Int J Cancer 1991 47: 252–260
Frei E III, Holden SA, Gonin R et al. Antitumor alkylating agents: in vitro cross-resistance and collateral sensitivity studies Cancer Chemother Phamacol 1993 33: 113–122
Elias AD, Richardson P, Avigan D et al. A short course of induction chemotherapy followed by two cycles of high-dose chemotherapy with stem cell rescue for chemotherapy naive metastatic breast cancer Bone Marrow Transplant 2001 (in press)
Webb IJ, Eickhoff CE, Elias A et al. Kinetics of peripheral blood mononuclear cell mobilization with chemotherapy and/or granulocyte-colony stimulating factor (G-CSF): implications for timing and yield of hematopoietic progenitor cell collections Transfusion 1996 36: 160–167
Hong RW, Rounds JD, Helton WS et al. Glutamine preserves liver glutathione after lethal hepatic injury Ann Surg 1992 215: 114–119
Comcowich SA, Spitzer TR, Tsunoda SM . Ursodiol to prevent hepatic venoocclusive disease Ann Pharmacother 1997 31: 1249–1252
George SL, Desu MM . Planning the size and duration of a clinical trial studying the time to some critical event J Chronic Dis 1974 27: 15–24
O'Brien PC, Fleming TR . A multiple testing procedure for clinical trials Biometrics 1979 35: 549–556
Cox DF . Analysis of Binary Data Chapman and Hall: London 1970
Kaplan EL, Meier R . Nonparametric estimation from incomplete observation J Am Stat Assoc 1958 53: 457–481
Postma TJ, Vermorken JB, Liefting AJ et al. Paclitaxel-induced neuropathy Ann Oncol 1995 6: 489–494
Rahman ZU, Frye DK, Buzdar AU et al. Impact of selection process on response rate and long-term survival of potential high-dose chemotherapy candidates treated with standard-dose doxorubicin-containing chemotherapy in patients with metastatic breast cancer J Clin Oncol 1997 15: 3171–3177
Garcia-Carbonero R, Hidalgo M, Paz-Ares L et al. Patient selection in high-dose chemotherapy trials: relevance in high-risk breast cancer J Clin Oncol 1997 15: 3178–3184
Berry DA, Broadwater G, Perry MC et al. Conventional- vs high-dose therapy for metastatic breast cancer: comparison of Cancer and Leukemia Group B (CALGB) and Blood and Marrow Transplant Registry (ABMTR) patients Proc ASCO 1999 18: 128a (A-490)
Antman K, Ayash L, Elias A et al. A phase II study of high dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard dose therapy J Clin Oncol 1992 10: 102–110
Lotz JP, Cure H, Janvier M et al. High dose chemotherapy with hematopoietic stem cell transplantation for metastatic breast cancer: Results of the French protocol PEGASE 04 Proc ASCO 1999 18: 43a
Stadtmauer EA, O'Neill A, Goldstein LJ et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer New Engl J Med 2000 342: 1069–1076
Bezwoda WR, Seymour L, Dansey RD . High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial J Clin Oncol 1995 13: 2483–2489
Acknowledgements
This work was supported in part by a grant from the Public Health Service Grant CA13849 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elias, A., Richardson, P., Avigan, D. et al. A short course of induction chemotherapy followed by two cycles of high-dose chemotherapy with stem cell rescue for chemotherapy naive metastatic breast cancer: sequential phase I/II studies. Bone Marrow Transplant 28, 447–454 (2001). https://doi.org/10.1038/sj.bmt.1703148
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703148