Abstract
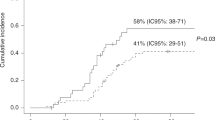
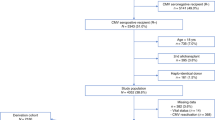
CMV pneumonia is a major cause of morbidity and mortality among allogeneic BMT recipients. To assess the frequency, timing, risk factors and response to therapy of CMV pneumonia among autologous BMT recipients, we reviewed our experience with 795 patients. Sixteen (2%) patients were diagnosed with CMV pneumonia. The frequency was higher among patients who were seropositive than those who were seronegative (3.3% vs 0%, P = 0.008). Among seropositive patients, the frequency was higher among patients with hematological malignancies than patients with solid tumors (5.0 % vs1.0%, P = 0.019). Eleven cases occurred <30 days, and five cases occurred >100 days post transplant. The overall CMV pneumonia-related mortality rate was 31%. Seven (78%) of nine patients treated with ganciclovir and IVIG prior to respiratory failure survived; neither of two patients treated after respiratory failure survived. Four of five (80%) untreated patients survived. In conclusion, CMV is a not infrequent cause of pneumonia among autologous BMT recipients. Risk factors include CMV seropositivity and an underlying hematological malignancy. A favorable response hinges on the prompt initiation of therapy. The survival of 25% of the patients without antiviral therapy suggests that the isolation of CMV from a BAL specimen occasionally reflects oropharyngeal contamination or that CMV pneumonia may sometimes be self-limited in more immunocompetent autologous BMT recipients. Bone Marrow Transplantation (2001) 27, 877–881.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pecego R, Hill R, Appelbaum FR et al. Interstitial pneumonitis following autologous bone marrow transplantation Transplantation 1986 42: 515–517
Valteau D, Hartmann O, Benhamou E et al. Nonbacterial nonfungal interstitial pneumonitis following autologous bone marrow transplantation in children treated with high-dose chemotherapy without total-body irradiation Transplantation 1988 45: 737–740
Wingard JR, Chen DY, Burns WH et al. Cytomegalovirus infection after autologous bone marrow transplantation with comparison to infection after allogeneic bone marrow transplantation Blood 1988 71: 1432–1437
Wingard JR, Sostrin MB, Vriesendorp HM et al. Interstitial pneumonitis following autologous bone marrow transplantation Transplantation 1988 46: 61–65
Reusser P, Fisher LD, Buckner CD et al. Cytomegalovirus infection after autologous bone marrow transplantation: occurrence of cytomegalovirus disease and effect on engraftment Blood 1990 75: 1888–1894
Enright H, Haake R, Weisdorf D et al. Cytomegalovirus pneumonia after bone marrow transplantation. Risk factors and response to therapy Transplantation 1993 55: 1339–1346
Ljungman P, Biron P, Bosi A et al. Cytomegalovirus interstitial pneumonia in autologous bone marrow transplant recipients. Infectious Disease Working Party of the European Group for Bone Marrow Transplantation Bone Marrow Transplant 1994 13: 209–212
Boeckh M, Gooley TA, Reusser P et al. Failure of high-dose acyclovir to prevent cytomegalovirus disease after autologous marrow transplantation J Infect Dis 1995 172: 939–943
Boeckh M, Stevens-Ayers T, Bowden RA . Cytomegalovirus pp65 antigenemia after autologous marrow and peripheral blood stem cell transplantation J Infect Dis 1996 174: 907–912
Singhal, R Powles, J Treleaven et al. Cytomegaloviremia after autografting for leukemia: clinical significance and lack of effect on engraftment Leukemia 1997 11: 835–838
Holmberg LA, Boeckh M, Hooper H et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation Blood 1999 94: 4029–4035
Bilgrami S, Aslanzadeh J, Feingold JM et al. Cytomegalovirus viremia, viruria and disease after autologous peripheral blood stem cell transplantation: no need for surveillance Bone Marrow Transplant 1999 24: 69–73
Offidani M, Corvatta L, Olivieri A et al. Infectious complications after autologous peripheral blood progenitor cell transplantation followed by G-CSF Bone Marrow Transplant 1999 24: 1079–1087
Bowden RA, Sayers M, Flournoy N et al. Cytomegalovirus immune globulin and seronegative blood products to prevent primary cytomegalovirus infection after marrow transplantation New Engl J Med 1986 314: 1006–1010
Verdonck LF, de Graan-Hentze YCE, Dekker AW et al. Cytomegalovirus seronegative platelets and leukocyte-poor red blood cells from random donors can prevent primary cytomegalovirus infection after bone marrow transplantation Bone Marrow Transplant 1987 2: 73–78
DeWitte T, Schattenberg A, Van Dijk BA et al. Prevention of primary cytomegalovirus infection after allogeneic bone marrow transplantation by using leukocyte-poor random blood products from cytomegalovirus unscreened blood-bank donors Transplantation 1990 50: 964–968
Bowden RA, Slichter SJ, Sayers MH et al. Use of leukocyte-depleted platelets and cytomegalovirus-seronegative red blood cells for prevention of primary cytomegalovirus infection after marrow transplant Blood 1991 78: 246–250
Whimbey E, Champlin RE, Couch RB et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients Clin Infect Dis 1996 22: 778–782
Crawford SW, Bowden RA, Hackman RC et al. Rapid detection of cytomegalovirus pulmonary infection by bronchoalveolar lavage and centrifugation culture Ann Intern Med 1988 108: 180–185
Mann M, Shelhamer JH, Masur H et al. Lack of clinical utility of bronchoalveolar lavage cultures for cytomegalovirus in HIV infection Am J Respir Crit Care Med 1997 155: 1723–1728
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Konoplev, S., Champlin, R., Giralt, S. et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplant 27, 877–881 (2001). https://doi.org/10.1038/sj.bmt.1702877
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702877
Keywords
This article is cited by
-
Survey of HCMV in allogenic and autologous stem cell transplantation by real-time PCR in Kermanshah, west of Iran
Infectious Agents and Cancer (2021)
-
Pneumonien bei immunsupprimierten Patienten
Der Radiologe (2017)
-
Cytomegalovirus infection in patients with haematological diseases and after autologous stem cell transplantation as consolidation: a single-centre study
Annals of Hematology (2017)
-
Cytomegalovirus infection in autologous stem cell transplant recipients in the era of rituximab
Annals of Hematology (2016)
-
Impaired pulmonary immunity post-bone marrow transplant
Immunologic Research (2011)