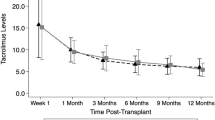
Abstract
for prevention of graft-versus-host disease, the consensus initial intravenous dose of tacrolimus for adults is 0.03 mg/kg/day. whether target whole blood concentrations of tacrolimus in children undergoing hematopoietic stem cell transplantation can be achieved reproducibly with this dose is not known. we reviewed the tacrolimus blood levels and calculated clearances for 55 children (aged 6 months to 18 years, median 9 years) using tacrolimus after allogeneic marrow, blood stem cell or cord blood transplantation. the tacrolimus dose regimen was 0.03 mg/kg/day by continuous infusion starting on day −1 or day −2. at the first sampling in the peritransplant period, 71% of the tacrolimus blood levels were within the target range of 5–15 ng/ml, 87% were in the safe range of 5–20 ng/ml, 9% were toxic, and 4% were subtherapeutic. twenty-five children were converted to oral drug using the recommended oral/intravenous dose ratio of 4.0. at the first sampling after oral conversion, 80% were in the target range, and 20% were subtherapeutic. clearance of tacrolimus was calculated from the blood levels for patients during intravenous dosing and normalized by ideal body weight. there was a decreased clearance over the first 2 weeks only for the children >12 years old (P = 0.014). The initial calculated clearances of tacrolimus did not differ between age groups, but at steady state the mean tacrolimus clearance (± s.d.) was higher for those <6 years old (0.159 ± 0.082 l/h/kg) than for those 6–12 years old (0.109 ± 0.053 l/h/kg) or >12 years old (0.104 ± 0.068 l/h/kg). Children <6 years old undergoing hematopoietic stem cell transplantation have a higher weight-normalized tacrolimus clearance than older children and adults, and careful therapeutic monitoring is needed in the first 2 weeks after transplantation to avoid prolonged subtherapeutic dosing for this age group. Bone Marrow Transplantation (2000) 26, 601–605.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ratanatharathorn V, Nash RA, Przepiorka D et al. Phase III study comparing methotrexate and tacrolimus (Prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation Blood 1998 92: 2303–2314
Nash RA, Antin JH, Karanes C et al. A phase III study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors Blood 1997 90: 561a (Abstr. 2499)
Hiraoka AF for the Japanese FK506 BMT Study Group . Results of a phase III study on prophylactic use of FK506 for acute GVHD compared with cyclosporine in allogeneic bone marrow transplantation Blood 1997 90: 561a (Abstr. 2500)
Przepiorka D, Devine SM, Fay JW et al. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation Bone Marrow Transplant 1999 24: 1053–1056
Koehler MT, Howrie D, Mirro J et al. FK506 (tacrolimus) in the treatment of steroid-resistant acute graft-versus-host disease in children undergoing bone marrow transplantation Bone Marrow Transplant 1995 15: 895–899
Przepiorka D, Petropoulos D, Mullen CA et al. Tacrolimus for prevention of graft-versus-host disease after mismatched unrelated donor cord blood transplantation Bone Marrow Transplant 1999 23: 1291–1295
Worth LL, Tran H, Petropoulos D et al. Hematopoietic stem cell transplantation for childhood myeloid malignancies after high-dose thiotepa, busulfan and cyclophosphamide Bone Marrow Transplant 1999 24: 947–952
Jain AB, Fung JJ, Tzakis AG et al. Comparative study of cyclosporine and FK506 dosage requirements in adult and pediatric orthotopic liver transplant patients Transplant Proc 1991 23: 2763–2766
McDiarmid SV, Colonna JO, Shaked A et al. Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients Transplantation 1993 55: 1328–1332
Robinson BV, Boyle GJ, Miller SA et al. Optimal dosing of intravenous tacrolimus following pediatric heart transplantation J Heart Lung Transplant 1999 18: 786–791
Yasuhara M, Hashida T, Toraguchi M et al. Pharmacokinetics and pharmacodynamics of FK506 in pediatric patients receiving living-related donor liver transplantations Transplant Proc 1995 27: 1108–1110
Wallemacq PE, Furlan V, Moller A et al. Pharmacokinetics of tacrolimus (FK506) in pediatric liver transplant recipients Eur J Drug Metab Pharmacokinet 1998 23: 367–370
Grenier FC, Luczkiw J, Bergmann M et al. A whole blood FK506 assay for the IMx analyzer Transplant Proc 1991 23: 2748–2749
Mehta P, Beltz S, Kedar A et al. Increased clearance of tacrolimus in children: need for higher doses and earlier initiation prior to bone marrow transplantation Bone Marrow Transplant 1999 24: 1323–1327
Boswell GW, Bekersky I, Fay J et al. Tacrolimus pharmacokinetics in BMT patients Bone Marrow Transplant 1998 21: 23–28
Spencer CM, Goa KL, Gillis JC . Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation Drugs 1997 54: 925–975
Tanaka E . In vivo age-related changes in hepatic drug-oxidizing capacity in humans J Clin Pharm Ther 1998 23: 247–255
Murry DJ, Crom WR, Reddick WE et al. Liver volume as a determinant of drug clearance in children and adolescents Drug Metab Dispos 1995 23: 1110–1116
Wingard JR, Nash RA, Przepiorka D et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation Biol Blood Marrow Transplant 1998 4: 157–163
Przepiorka D, Nash RA, Wingard JR et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation Biol Blood Marrow Transplant 1999 5: 94–97
Przepiorka D, Saliba R, Cleary K et al. Tacrolimus does not abrogate the increased risk of acute graft-vs-host disease after unrelated-donor marrow transplantation with allelic mismatching at HLA-DRB1 and HLA-DQB1 Biol Blood Marrow Transplant 2000 6: 190–197
Przepiorka D, Ippoliti C, Khouri I et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after matched unrelated donor marrow transplantation Blood 1996 88: 4383–4389
Woo MH, Przepiorka D, Ippoliti C et al. Toxicities of tacrolimus and cyclosporine after allogeneic blood stem cell transplantation Bone Marrow Transplant 1997 20: 1095–1098
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Przepiorka, D., Blamble, D., Hilsenbeck, S. et al. Tacrolimus clearance is age-dependent within the pediatric population. Bone Marrow Transplant 26, 601–605 (2000). https://doi.org/10.1038/sj.bmt.1702588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702588
Keywords
This article is cited by
-
Discussion on machine learning technology to predict tacrolimus blood concentration in patients with nephrotic syndrome and membranous nephropathy in real-world settings
BMC Medical Informatics and Decision Making (2022)
-
Influence of melphalan plus fludarabine-conditioning regimen in elderly patients aged ⩾55 years with hematological malignancies
Bone Marrow Transplantation (2016)
-
Pharmacokinetics, Pharmacodynamics and Pharmacogenomics of Immunosuppressants in Allogeneic Haematopoietic Cell Transplantation: Part I
Clinical Pharmacokinetics (2016)
-
Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid-resistant nephrotic syndrome
Pediatric Nephrology (2015)
-
Effect of CYP3A5 genotype, steroids, and azoles on tacrolimus in a pediatric renal transplant population
Pediatric Nephrology (2014)