Abstract
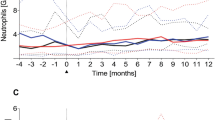
The potent immunostimulatory cytokine interleukin-2 (IL-2) has been extensively investigated for its potential to induce anti-tumor immunity in a number of tumor models. Only recently the complex interplay of mutually suppressive or supportive cytokines of the IL-2-induced network of cytokines has been better characterized. The aim of this study was to assess which of these in vitro findings are reproducible in vivo in recipients of stem cell transplants (SCT), since in these patients long- lasting impairments in cytokine inducibility have been described. We have therefore studied the kinetics of putative modulators and mediators of IL-2-induced immune activation, namely IL-1β, IL-4, IL-5, IL-10, IL-12, soluble Fas ligand (sFasL), and GM-CSF during IL-2 therapy. All patients were children or adolescents suffering from solid tumors with poor prognosis who received three 5-day courses of high-dose intravenous IL-2 as an adjuvant to their radio-chemotherapy and autologous SCT. While IL-1β, IL-4 and IL-12 were not, and sFasL was only mildly affected by the IL-2 therapy, we observed a consistent and early rise of IL-10, IL-5, and GM-CSF. These increases were rapidly reversible after discontinuation of IL-2 therapy. The inducibility of IL-10, IL-5 and GM-CSF was more pronounced with increasing time from the SCT, and in the third cycle reached an order of magnitude as in high-dose IL-2 patients without SCT. Together with the abundant in vitrodata, these findings may help devise a combination immunotherapy permitting stronger anti-tumor effects, but lesser adverse effects. Bone Marrow Transplantation (2000) 26, 91–96.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bukowski RM, McLain D, Olencki T et al. Interleukin-2: use in solid tumors Stem Cells (Dayt) 1993 11: 26–32
Guillaume T, Sekhavat M, Rubinstein DB et al. Defective cytokine production following autologous stem cell transplantation for solid tumors and hematologic malignancies regardless of bone marrow or peripheral origin and lack of evidence for a role for interleukin-10 in delayed immune reconstitution Cancer Res 1994 54: 3800–3807
Hanenberg H, Dilloo D, Laws HJ et al. Time course of interferon-gamma production deficiency after autologous and allogeneic stem cell transplantation for malignancies Exp Hematol 1995 23: 1543–1552
Engelhardt M, Wirth K, Mertelsmann R et al. Clinical and immunomodulatory effects of repetitive 2-day cycles of high-dose continuous infusion IL-2 Eur J Cancer 1997 33: 1050–1054
Tilg H, Atkins MB, Dinarello CA et al. Induction of circulating interleukin 10 by interleukin 1 and interleukin 2, but not interleukin 6 immunotherapy Cytokine 1995 7: 734–739
Dilloo D, Laws HJ, Hanenberg H et al. Induction of two dinstinct natural killer-cell populations, activated T cells and antineoplastic cytokines, by interleukin-2 therapy in children with solid tumors Exp Hematol 1994 22: 1081–1088
Heslop HE, Duncombe AS, Reittie JE et al. Interleukin 2 infusion induces haemopoietic growth factors and modifies marrow regeneration after chemotherapy of autologous marrow transplantation Br J Haematol 1991 77: 237–244
Tagliaferri P, Barile C, Caraglia M et al. Daily low-dose subcutaneous recombinant interleukin-2 by alternate weekly administration: antitumor activity and immunomodulatory effects Am J Clin Oncol 1998 21: 48–53
Mier JW, Vachino G, van der Meer JW et al. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients J Clin Immunol 1988 8: 426–436
Lorenz HM, Hieronymus T, Grünke M et al. Differential role for IL-2 and IL-15 in the inhibition of apoptosis in short-term activated human lymphocytes Scand J Immunol 1997 45: 660–669
Saraya KA, Balkwill FR . Temporal sequence and cellular origin of interleukin-2 stimulated cytokine gene expression Br J Cancer 1993 67: 514–521
Heslop HE, Gottlieb DJ, Bianchi AC et al. In vivo induction of gamma interferon and tumor necrosis factor by interleukin-2 infusion following intensive chemotherapy or autologous marrow transplantation Blood 1989 74: 1374–1380
Körholz D, Banning U, Bönig H et al. The role of interleukin 10 (IL-10) in IL-15 mediated T-cell responses Blood 1997 90: 4513–4521
Guillaume T, Kubin M, Sekhavat M et al. Peripheral blood mononuclear cells from autologous hematopoietic stem cell transplantation recipients produce and respond to IL-12 Bone Marrow Transplant 1996 18: 733–739
Girard D, Gosselin J, Heitz D et al. Effects of interleukin-2 on gene expression in human neutrophils Blood 1995 86: 1170–1176
Schaafsma MR, Falkenburg JHF, Landegent JE et al. In vivo production of interleukin-5, granulocyte–macrophage colony-stimulating factor, and interleukin-6 during intravenous administration of high-dose interleukin-2 in cancer patients Blood 1991 78: 1981–1987
VanHaelst Pisani C, Kovach JS, Kita H et al. Administration of interleukin-2 (IL-2) results in increased plasma concentrations of IL-5 and eosinophilia in patients with cancer Blood 1991 78: 1538–1544
Tritarelli E, Rocca E, Testa U et al. Adoptive immunotherapy with high-dose interleukin-2: kinetics of circulating progenitors correlate with interleukin-6, granulocyte colony-stimulating factor level Blood 1991 77: 741–749
Sosman JA, Bartemes K, Offord KP et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: effects of interleukin-4 alone and following interleukin-2 administration Clin Cancer Res 1995 1: 805–812
Burdach St, Jürgens H, Peters C et al. Myeloablative chemo-radiotherapy and hematopoietic stem cell rescue in poor prognosis Ewing's sarcoma J Clin Oncol 1993 11: 1482–1488
Hempel L, Körholz D, Nussbaum P et al. High interleukin-10 serum levels are associated with fatal outcome in patients after bone marrow transplantation Bone Marrow Transplant 1997 20: 365–368
Lissoni P, Fumagalli L, Rovelli F et al. In vivo stimulation of IL-12 secretion by subcutaneous low-dose IL-2 in metastatic cancer patients Br J Cancer 1998 77: 1957–1960
Gottlieb DJ, Prentice HG, Heslop HE et al. IL-2 infusion abrogates humoral immune responses in humans Clin Exp Immunol 1992 87: 493–498
Bönig H, Hannen M, Lex C et al. Additive effects of infection and neutropenia on the induction of granulocytopoietic activity in vivo Cancer 1999 86: 340–348
Tilden AB, Dunlap NE . Interleukin-2 augmentation of interleukin-1 and prostaglandin E2 production J Leuk Biol 1989 45: 474–477
Verheyen J, Bönig H, Kim YM et al. Regulation of interleukin-2 induced interleukin-5 and interleukin-13 production in human peripheral blood mononuclear cells Scand J Immunol 2000 51: 45–51
Acknowledgements
This study was supported by the Elterninitiative Kinderkrebsklinik eV, Düsseldorf, Germany, and the German Cancer Research Fund, Bonn, Germany. An abstract covering parts of these data was presented at the 1999 ISEH meeting in Monte Carlo, Monaco.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bönig, H., Laws, HJ., Wundes, A. et al. In vivo cytokine responses to interleukin-2 immunotherapy after autologous stem cell transplantation in children with solid tumors. Bone Marrow Transplant 26, 91–96 (2000). https://doi.org/10.1038/sj.bmt.1702431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702431