Abstract
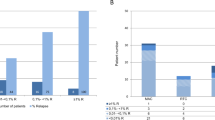
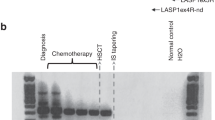
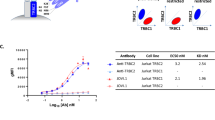
One of the major problems after allogeneic bone marrow transplantation (BMT) is a high frequency of leukemia relapse. We have prospectively studied the presence of donor- and recipient-derived chimeric cells in bone marrow recipients with pre-B cell acute lymphoblastic leukemia (pre-B-ALL). The chimeric status of BMT recipients was compared to minimal residual disease (MRD) detection by analysis of immunoglobulin heavy chain (IgH) and T cell receptor (TcR) genes. Post-transplant blood and bone marrow samples from 12 patients with pre-B-ALL were studied. Five patients showed mixed chimerism (MC) in the CD19-positive cell fraction. Four of them have relapsed to date. The remaining patient with MC in the B cell lineage was also MRD positive in the same samples. All seven patients with donor chimerism in the B cell fraction remain in clinical remission (P = 0.01). In samples from all five patients having MC in the B cell lineage, the patient-specific IgH or TcR rearrangement was also detected. In three of four patients who relapsed, MC in the B cell lineage was seen more than 2.5 months prior to morphologically verified relapse. The results of this comparison suggest that routinely performed MC analysis of the affected cell lineage may facilitate post-BMT monitoring and rapid therapeutic decisions in transplanted patients with pre-B-ALL. Bone Marrow Transplantation (2000) 25, 843–851.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thomas ED, Sanders JE, Flournoy N et al. Marrow transplantation for patients with acute lymphoblastic leukemia in remission Blood 1979 54: 468–476
Barrett AJ, Horowitz MM, Pollock BH et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission New Engl J Med 1994 331: 1253–1258
Saarinen UM, Mellander L, Nysom K et al. Allogeneic bone marrow transplantation in first remission for children with very high-risk acute lymphoblastic leukemia: a retrospective case-control study in the Nordic countries. Nordic Society for Pediatric Hematology and Oncology (NOPHO) Bone Marrow Transplant 1996 17: 357–363
Burnett AK, Eden OB . The treatment of acute leukaemia Lancet 1997 349: 270–275
Nizet Y, Van Daele S, Lewalle P et al. Long-term follow-up of residual disease in acute lymphoblastic leukemia patients in complete remission using clonogeneic IgH probes and the polymerase chain reaction Blood 1993 82: 1618–1625
Roberts WM, Estrov Z, Ouspenskaia MV et al. Measurement of residual leukemia during remission in childhood acute lymphoblastic leukemia New Engl J Med 1997 336: 317–323
Alard P, Matriano JA, Socarras S et al. Detection of donor-derived cells by polymerase chain reaction in neonatally tolerant mice. Microchimerism fails to predict tolerance Transplantation 1995 60: 1125–1130
Radich JP, Gehly G, Gooley T et al. Polymerase chain reaction detection of the BCR-ABL fusion transcript after allogeneic marrow transplantation for chronic myeloid leukemia: results and implications in 346 patients Blood 1995 85: 2632–2638,
Norris MD, Kwan E, Haber M, Marshall GM . Detection of evolving immunoglobulin heavy-chain gene rearrangements in acute lymphoblastic leukemia: a PCR-based assay employing overlapping DJH primers Leukemia 1995 9: 1779–1782
Burlingham WJ . Chimerism after organ transplantation: is there any clinical significance? Clin Transplant 1996 10: 110–117
Mackinnon S, Barnett L, Heller G . Polymerase chain reaction is highly predictive of relapse in patients following T cell-depleted allogeneic bone marrow transplantation for chronic myeloid leukemia Bone Marrow Transplant 1996 17: 643–647
Yokota S, Hansen-Hagge TE, Bartram CR . T-cell receptor delta gene recombination in common acute lymphoblastic leukemia: preferential usage of V delta 2 and frequent involvement of the J alpha cluster Blood 1991 77: 141–148
Beishuizen A, Verhoeven MA, van Wering ER et al. Analysis of Ig and T-cell receptor genes in 40 childhood acute lymphoblastic leukemias at diagnosis and subsequent relapse: implications for the detection of minimal residual disease by polymerase chain reaction analysis Blood 1994 83: 2238–2247
Hagelberg E, Gray IC, Jeffreys AJ . Identification of the skeletal remains of a murder victim by DNA analysis Nature 1991 352: 427–429
Ugozzoli L, Yam P, Petz LD et al. Amplification by the polymerase chain reaction of hypervariable regions of the human genome for evaluation of chimerism after bone marrow transplantation Blood 1991 77: 1607–1615
Mackinnon S, Barnett L, Bourhis JH et al. Myeloid and lymphoid chimerism after T-cell-depleted bone marrow transplantation: evaluation of conditioning regimens using the polymerase chain reaction to amplify human minisatellite regions of genomic DNA Blood 1992 80: 3235–3241
Steward CG, Goulden NJ, Katz F et al. A polymerase chain reaction study of the stability of Ig heavy-chain and T-cell receptor delta gene rearrangements between presentation and relapse of childhood B-lineage acute lymphoblastic leukemia Blood 1994 83: 1355–1362
Olerup O, Zetterquist H . HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor–recipient matching in cadaveric transplantation Tissue Antigens 1992 39: 225–235
Ringdén O, Remberger M, Persson U et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings Bone Marrow Transplant 1995 15: 619–625
Oakhill A, Pamphilon DH, Potter MN et al. Unrelated donor bone marrow transplantation for children with relapsed acute lymphoblastic leukaemia in second complete remission Br J Haematol 1996 94: 574–578
Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia New Engl J Med 1986 314: 729–735
Ringden O, Pihlstedt P, Markling L et al. Prevention of graft-versus-host disease with T cell depletion or cyclosporin and methotrexate. A randomized trial in adult leukemic marrow recipients Bone Marrow Transplant 1991 7: 221–226
Budowle B, Chakraborty R, Giusti AM et al. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE Am J Hum Genet 1991 48: 137–144
Horn GT, Richards B, Klinger KW . Amplification of a highly polymorphic VNTR segment by the polymerase chain reaction Nucleic Acids Res 1989 17: 2140
Eubanks JH, Selleri L, Hart R et al. Isolation, localization, and physical mapping of a highly polymorphic locus on human chromosome 11q13 Genomics 1991 11: 720–729
Decorte R, Cuppens H, Marynen P, Cassiman JJ . Rapid detection of hypervariable regions by the polymerase chain reaction technique DNA Cell Biol 1990 9: 461–469
Buchmayer H, Rumpold H, Mannhalter C . Identification of a variable number tandem repeat region in the human T cell receptor alpha-delta (TCRAD) locus Hum Genet 1996 98: 333–335
Jeffreys AJ, Wilson V, Neumann R, Keyte J . Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells Nucleic Acids Res 1988 16: 10953–10971
Yamada M, Hudson S, Tournay O et al. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes Proc Natl Acad Sci USA 1989 86: 5123–5127
Steward CG, Goulden NJ, Potter MN, Oakhill A . The use of the polymerase chain reaction to detect minimal residual disease in childhood acute lymphoblastic leukaemia Eur J Cancer 1993 29A: 1192–1198
van Dongen JJ, Breit TM, Adriaansen HJ et al. Detection of minimal residual disease in acute leukemia by immunological marker analysis and polymerase chain reaction Leukemia 1992 1: 47–59
Kaplan E, Meier P . Nonparametric estimation from incomplete observations J Am Stat Assoc 1958 53: 457–481
Kalbfleisch J, Prentice R . The Statistical Analysis of Failure Time Data John Wiley and Sons: New York 1980
Ramirez M, Diaz MA, Garcia-Sanchez F et al. Chimerism after allogeneic hematopoietic cell transplantation in childhood acute lymphoblastic leukemia Bone Marrow Transplant 1996 18: 1161–1165
Bader P, Beck J, Frey A et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT Bone Marrow Transplant 1998 21: 487–495
Estrov Z, Ouspenskaia MV, Felix EA et al. Persistence of self-renewing leukemia cell progenitors during remission in children with B-precursor acute lymphoblastic leukemia Leukemia 1994 8: 46–52
Roux E, Abdi K, Speiser D et al. Characterization of mixed chimerism in patients with chronic myeloid leukemia transplanted with T-cell-depleted bone marrow: involvement of different hematologic lineages before and after relapse Blood 1993 81: 243–248
van Leeuwen JE, van Tol MJ, Joosten AM et al. Mixed T-lymphoid chimerism after allogeneic bone marrow transplantation for hematologic malignancies of children is not correlated with relapse Blood 1993 82: 1921–1928
Moos M, Schulz R, Cremer F et al. Detection of minimal residual disease by polymerase chain reaction in B cell malignancies Stem Cells 1995 3: 42–51
Gardiner N, Lawler M, O'Riordan JM et al. Monitoring of lineage-specific chimaerism allows early prediction of response following donor lymphocyte infusions for relapsed chronic myeloid leukaemia Bone Marrow Transplant 1998 21: 711–719
Li HH, Gyllensten UB, Cui XF et al. Amplification and analysis of DNA sequences in single human sperm and diploid cells Nature 1988 335: 414–417
Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B . Host origin of marrow stromal cells following allogeneic bone marrow transplantation Nature 1987 328: 429–432
McCann SR, Lawler M . Mixed chimaerism; detection and significance following BMT Bone Marrow Transplant 1993 11: 91–94
Roux E, Helg C, Dumont-Girard F et al. Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts Blood 1996 87: 3984–3992
Lawler M, Humphries P, McCann SR . Evaluation of mixed chimerism by in vitro amplification of dinucleotide repeat sequences using the polymerase chain reaction Blood 1991 77: 2504–2514
Molloy K, Goulden N, Lawler M et al. Patterns of hematopoietic chimerism following bone marrow transplantation for childhood acute lymphoblastic leukemia from volunteer unrelated donors Blood 1996 87: 3027–3031
Green E, McConville CM, Powell JE et al. Clonal diversity of Ig and T-cell-receptor gene rearrangements identifies a subset of childhood B-precursor acute lymphoblastic leukemia with increased risk of relapse Blood 1998 92: 952–958
Knechtli CJ, Goulden NJ, Hancock JP et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia Blood 1998 92: 4072–4079
Langlands K, Goulden NJ, Steward CG et al. False-positive residual disease assessment after bone marrow transplant in acute lymphoblastic leukemia Blood 1994 84: 1352–1353
Campana D, Pui CH . Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance Blood 1995 85: 1416–1434
Kolb HJ, Mittermuller J, Clemm C et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients Blood 1990 76: 2462–2465
Bader P, Beck J, Schlegel PG et al. Additional immunotherapy on the basis of increasing mixed hematopoietic chimerism after allogeneic BMT in children with acute leukemia: is there an option to prevent relapse? Bone Marrow Transplant 1997 20: 79–81
Slavin S, Nagler A, Naparstek E et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases Blood 1998 91: 756–763
Acknowledgements
Dr Collin Steward and Dr Chris Knechtli, Bristol Royal Hospital for Sick Children, Bristol, UK and Dr Mike Potter, Royal Free Hospital, London, UK are gratefully acknowledged for generous technical advice. We are also indebted to Susanne Öhman for technical assistance. This study was supported by grants from the Swedish Cancer Foundation (0070-B93), the Childrens Cancer Foundation (1991-057) and the Swedish Medical Research Council (16X-05971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zetterquist, H., Mattsson, J., Uzunel, M. et al. Mixed chimerism in the B cell lineage is a rapid and sensitive indicator of minimal residual disease in bone marrow transplant recipients with pre-B cell acute lymphoblastic leukemia. Bone Marrow Transplant 25, 843–851 (2000). https://doi.org/10.1038/sj.bmt.1702337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702337
Keywords
This article is cited by
-
Chimerism analysis for clinicians: a review of the literature and worldwide practices
Bone Marrow Transplantation (2022)
-
Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies
Bone Marrow Transplantation (2009)
-
Monitoring EBV DNA in saliva for early diagnosis of EBV reactivation in solid tumour patients after allogeneic haematopoietic SCT
Bone Marrow Transplantation (2009)
-
B-cell reconstitution after allogeneic SCT impairs minimal residual disease monitoring in children with ALL
Bone Marrow Transplantation (2008)
-
Treatment options for the management of acute leukaemia relapsing following an allogeneic transplant
Bone Marrow Transplantation (2008)