Abstract
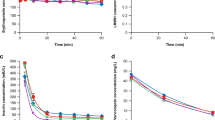
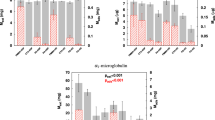
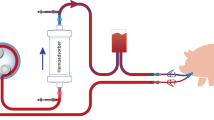
Two main factors that affect the pharmacokinetics of cyclosporin A (CsA) during 24-h durable intravenous (DIV) administration have been reported, namely physiological changes after bone marrow transplantation, and blood sampling through indwelling lines. In addition, it has been found that infusion sets made of polyvinyl chloride (PVC) markedly adsorb CsA. We conducted in vitro adsorption studies of CsA on infusion sets, and the administration routes that are used in the treatment of patients with bone marrow transplantation. We also examined the effects of administration route on CsA pharmacokinetics in clinical practice. The in vitro adsorption study using 30-mm segments of lumen from commercially available infusion sets showed that the degree of CsA adsorption per area of lumen made of PVC was significantly higher than that in those made of polyethylene (PE) or polybutadiene (PB), which showed no adsorption of CsA. Due to its adsorption, use of infusion sets made of PVC resulted in about a 40–50% loss of CsA dose, which affected the pharmacokinetic parameters during 24-h DIV, while those made of PE and PB did not. The use of non-PVC infusion sets should allow for accurate monitoring of CsA results, and provide cost benefit in the treatment of bone marrow transplantation. Bone Marrow Transplantation (2000) 25, 633–638.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Storb R, Deeg HJ, Whitehead J . Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia New Engl J Med 1986 314: 729–735
Azuma E, Kojima S, Kato K et al. Conditioning with cyclophosphamide/antithymocyte globulin for allogenic bone marrow transplantation from HLA-matched siblings in children with severe aplastic anemia Bone Marrow Transplant 1997 19: 1085–1087
Ptachcinski RJ, Venkataramanan R, Burckart GJ . Clinical pharmacokinetics of cyclosporin Clin Pharmacokinet 1986 11: 107–132
Shaw LM, Bowers L, Demers L et al. Clinical issues in cyclosporine monitoring: report of the task force on cyclosporine monitoring Clin Chem 1987 33: 1269–1288
Busca A, Miniero R, Vassallo E et al. Monitoring of cyclosporine blood levels from central venous lines: a misleading assay? Ther Drug Monit 1994 16: 71–74
Nakajima S, Kawano K, Nakazawa K, Terada K . The loss of cyclosporine from solutions into the administration set Byoin Yakugaku 1988 14: 335–338
Nakajima S, Kawano K, Nakazawa K . Loss of isosorbide dinitrate from solutions in administration set Yakuzaigaku 1985 45: 285–290
Nakajima S, Kawano K, Nakazawa K, Suzuki K . The loss of isosorbide dinitrate and that of nitroglycerin from solutions into the administration set Yakuzaigaku 1988 48: 204–208
Abbott Laboratories Diagnostic Division, TDx System Operation Manual, Abbott Laboratories, North Chicago 1984
Cockcroft DW, Gault MH . Prediction of creatinine clearance from serum creatinine Nephron 1976 16: 31–41
Sheiner LB, Beal SL . Some suggestions for measuring predictive performance J Pharmacokinet Biopharm 1981 9: 503–512
Shibata N, Minouchi T, Hayashi Y et al. Effect of temperature and endogenous factors in blood on concentration of cyclosporin in plasma measured by high-performance liquid chromatography Chem Pharm Bull 1989 37: 1877–1880
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shibata, N., Ikuno, Y., Tsubakimoto, Y. et al. Adsorption and pharmacokinetics of cyclosporin A in relation to mode of infusion in bone marrow transplant patients. Bone Marrow Transplant 25, 633–638 (2000). https://doi.org/10.1038/sj.bmt.1702196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702196
Keywords
This article is cited by
-
Evaluation of nitroglycerin and cyclosporin A sorption to polyvinylchloride- and non-polyvinylchloride-based tubes in administration sets
Journal of Pharmaceutical Investigation (2018)
-
Adsorption and Leachable Contamination of Flucloxacillin, Cyclosporin and Amiodarone Following Delivery Through an Intravenous Administration Set
Pharmaceutical Research (2018)
-
Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood
Critical Care (2015)