Abstract
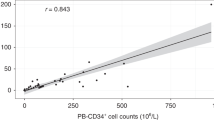
CD90 or Thy-1 is an antigen co-expressed with CD34+ on putative immature hematopoietic stem cells. Peak mobilization of CD34+90+ cells into the blood occurs a few days earlier than peak mobilization of total CD34+ cells. Because it is not known which cell type best correlates with engraftment, the optimal timing of apheresis remains unclear. The purpose of the study was to determine if the CD34+90+ cell dose predicts engraftment of autologous blood stem cells independent of the total CD34+ cell dose/kg, the dose of other CD34+ cell subsets (CD34+33−, CD34+38−, CD34+41+), or various clinical factors. Data were analyzed on 125 consecutive patients ranging in age from 19 to 66 years (median 46) who underwent autologous blood stem cell transplantation (ABSCT) for breast cancer (54), lymphoma (59), or other malignancies (12). By univariate analysis, neutrophil (⩾0.5 × 109/l) and platelet (⩾20 × 109/l or ⩾100 × 109/l) engraftment correlated better with the total CD34+ cell dose than with the CD34+90+ cell subset. Using Cox proportional hazards models, factors independently associated with both neutrophil engraftment (⩾0.5 × 109/l) and platelet engraftment (⩾20 × 109/l and ⩾100 × 109/l) were higher total CD34+ dose/kg and high-dose regimen (melphalan-containing slower than other regimens). In conclusion, the total CD34+ dose/kg was a better predictor of hematopoietic engraftment following ABSCT than the dose of any CD34+ subset, including CD34+90+ cells. Apheresis should continue to be timed according to peak CD34+ levels. Bone Marrow Transplantation (2000) 25, 435–440.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
To LB, Haylock DN, Simmons PJ et al. The biology and clinical use of blood stem cells Blood 1997 89: 2233–2258
Sutherland DR, Anderson L, Keeney M et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry J Hematother 1996 5: 213–226
Zimmerman TM, Lee WJ, Bender JG et al. Quantitative CD34+ analysis may be used to guide peripheral-blood stem cell harvest Bone Marrow Transplant 1995 9: 439–444
Krause DS, Fackler MJ, Civin CI et al. CD34: structure, biology, and clinical utility Blood 1996 87: 1–13
Lansdorp PM, Sutherland HJ, Eaves CJ . Selective expression of CD45 isoforms on functional subpopulations of CD34+ hematopoietic cells from human bone marrow J Exp Med 1990 172: 363–366
Terstappen LWMM, Huang S, Safford M et al. Sequential generation of hematopoietic colonies derived from single nonlineage-committed CD34+38− progenitor cells Blood 1991 77: 1218–1227
Bernstein ID, Leary AG, Andrews RG et al. Blast colony-forming cells and precursors of colony-forming cells detectable in long-term marrow culture express the same phenotype (CD33-CD34+) Exp Hematol 1991 19: 680–682
Pierelli L, Teofili L, Menichella G et al. Further investigations on the expression of HLA-DR, CD33, and CD13 surface antigens in purified bone marrow and peripheral blood CD34+ hematopoietic progenitor cells Br J Haematol 1994 84: 24–30
Brandt J, Baird N, Lu L et al. Characterization of a human hematopoietic progenitor cell capable of forming blast cell containing colonies in vitro J Clin Invest 1988 82: 1017–1027
Buhring HJ, Asenbauer B, Katrilaka K et al. Sequential expression of CD34 and CD33 antigens on myeloid colony-forming cells Eur J Haematol 1989 42: 143–149
Loken MR, Shah VO, Dattilio KL et al. Flow cytometric analysis of human bone marrow. I. Normal erythroid development Blood 1987 69: 255–263
Loken MR, Shah VO, Dattilio KL et al. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development Blood 1987 70: 1316–1324
Debili N, Issaad C, Masse JM et al. Expression of CD34 and platelet glycoproteins during human megakaryocytic differentiation Blood 1992 80: 3022–3035
Vainchenker W, Deschamps JF, Bastin JM et al. Two monoclonal antiplatelet antibodies as markers of human megakaryocyte maturation: immunofluorescent staining and platelet peroxidase detection in megakaryocyte colonies and in in vivo cells from normal and leukemic patients Blood 1982 59: 514–521
Stewart DA, Guo D, Luider J et al. Factors predicting engraftment of autologous blood stem cells: CD34+ subsets inferior to the total CD34+ cell dose Bone Marrow Transplant 1999 23: 1237–1243
Derksen MW, Rodenhuis S, Dirkson MKA et al. Subsets of CD34+ cells and rapid hematopoietic recovery after peripheral-blood stem cell transplantation J Clin Oncol 1995 13: 1922–1932
Millar BC, Millar JL, Shepherd V et al. The importance of CD34+/CD33− cells in platelet engraftment after intensive therapy for cancer patients given peripheral blood stem cell rescue Bone Marrow Transplant 1998 22: 469–475
Copelan EA, Ceselski SK, Ezzone SA et al. Mobilization of peripheral-blood progenitor cells with high dose etoposide and granulocyte colony-stimulating factor in patients with breast cancer, non-Hodgkin's lymphoma, and Hodgkin's disease J Clin Oncol 1997 15: 759–765
Pecora AL, Preti RA, Gleim GW et al. CD34+33− cells influence days to engraftment and transfusion requirements in autologous blood stem-cell recipients J Clin Oncol 1998 16: 2093–2104
Gonzalez-Requejo A, Madero L, Diaz MA et al. Progenitor cell subsets and engraftemnt kinetics in children undergoing autologous peripheral blood stem cell transplantation Br J Haematol 1998 101: 104–110
Craig W, Kay R, Cutler RL, Lansdorp PM . Expression of Thy-1 on human hematopoietic progenitor cells J Exp Med 1993 177: 1331–1342
Humeau L, Bardin F, Maroc C et al. Phenotypic, molecular and functional characterisation of human peripheral blood CD34+/Thy1+ cells Blood 1996 87: 949–955
Haas R, Mohle R, Murea S et al. Characterisation of peripheral blood progenitor cells mobilised by cytotoxic chemotherapy and recombinant granulocyte colony-stimulating factor J Hematother 1994 3: 323–330
Murray L, Chen B, Galy A et al. Enrichment of human hematopoietic stem cell activity in the CD34+Thy-1+Lin− subpopulation from mobilized peripheral blood Blood 1995 85: 368–378
Stewart AK, Keating IA, Anania S et al. Optimising the CD34+ and Thy-1+ stem cell content of peripheral blood collections Exp Hematol 1995 23: 1619–1627
Tsuchiya S, Kikuta A, Shimizu Y et al. Decrease in Thy-1 expression on peripheral CD34 positive cells induced by G-CSF mobilization. The Tohoku Children Leukemia Study Group Tohoku J Exp Med 1997 182: 157–162
Stewart DA, Guo D, Morris D et al. Superior autologous blood stem cell mobilization from dose-intensive cyclophosphamide, etoposide, cisplatin plus G-CSF than from less intensive chemotherapy regimens Bone Marrow Transplant 1999 23: 111–117
Bensinger W, Appelbaum F, Rowley S et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells J Clin Oncol 1995 13: 2547–2555
Weaver CH, Hazelton B, Birch R et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral-blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy Blood 1995 10: 3961–3969
Acknowledgements
We thank Jan McLaughlin for data collection and management.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stewart, D., Guo, D., Luider, J. et al. The CD34+90+ cell dose does not predict early engraftment of autologous blood stem cells as well as the total CD34+ cell dose. Bone Marrow Transplant 25, 435–440 (2000). https://doi.org/10.1038/sj.bmt.1702171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702171
Keywords
This article is cited by
-
Genome-wide association study identifies variants at 16p13 associated with survival in multiple myeloma patients
Nature Communications (2015)
-
Pegfilgrastim successfully mobilizes megakaryocyte progenitors into the peripheral blood in subjects with solid tumours
Bone Marrow Transplantation (2008)
-
Subsets of CD34+ and early engraftment kinetics in allogeneic peripheral SCT for AML
Bone Marrow Transplantation (2008)
-
Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers
Bone Marrow Transplantation (2005)
-
Impact of CD34 subsets on engraftment kinetics in allogeneic peripheral blood stem cell transplantation
Bone Marrow Transplantation (2005)