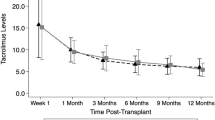
Abstract
The pharmacokinetics of tacrolimus have been studied in healthy volunteers and in adults undergoing bone marrow transplantation. However, there is little information on the pharmacokinetics of tacrolimus in children undergoing BMT. We studied pharmacokinetics of tacrolimus in seven patients (age 8–17 years) undergoing allogeneic stem cell transplantation. Four patients received matched unrelated donor (MUD) transplants, two underwent HLA-matched related donor transplants, and one underwent an umbilical cord blood donor transplant. All patients received tacrolimus by continuous infusion at 0.03–0.04 mg/kg/day beginning on the day prior to transplant. Tacrolimus whole blood concentrations were monitored by microparticle enzyme immunoassay. Our goal was to maintain a blood tacrolimus level of 10–20 μg/ml. Once patients were tolerating oral medications, tacrolimus infusion was converted to oral dosing using a 4:1 conversion. Dose of tacrolimus and resulting tacrolimus concentrations were recorded and the total body clearance of tacrolimus was calculated retrospectively. The mean clearance, based on first steady-state tacrolimus concentrations necessary for achieving a therapeutic level (10–20 μg/ml), was 108.1 ml/h/kg (range 79.7–142.0 ml/h/kg), greater than that reported in adult BMT patients (71 ± 34 ml/h/kg). The average dose required to achieve that therapeutic range was 0.0354 mg/kg/day as an intravenous continuous infusion. Over the entire course of intravenous tacrolimus, mean clearance was 97.0 ml/h/kg (range 33.4–153.3 ml/h/kg). In six of the seven patients, clearance values dropped after 2–4 weeks of therapy by an average of 32.5 ml/h/kg. In two patients, sharp drops in clearance were temporally related to changes in liver function tests. Three of the seven patients died of severe acute GVHD; all these had undergone matched unrelated donor transplantation, and two of these three had initial clearance levels over 120 ml/h/kg. Thus, children appear to have more rapid tacrolimus clearance than adults and may need to begin therapy earlier in order to obtain stable and optimal levels. More studies are needed to confirm these preliminary results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehta, P., Beltz, S., Kedar, A. et al. Increased clearance of tacrolimus in children: need for higher doses and earlier initiation prior to bone marrow transplantation. Bone Marrow Transplant 24, 1323–1327 (1999). https://doi.org/10.1038/sj.bmt.1702053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702053
Keywords
This article is cited by
-
Pharmacokinetics, Pharmacodynamics and Pharmacogenomics of Immunosuppressants in Allogeneic Haematopoietic Cell Transplantation: Part I
Clinical Pharmacokinetics (2016)
-
Pharmacokinetics for once-daily modified release formulation of tacrolimus hydrate in unrelated hematopoietic stem cell transplantation
Annals of Hematology (2015)
-
Engraftment syndrome, but not acute GVHD, younger age, CYP3A5 or MDR1 polymorphisms, increases tacrolimus clearance in pediatric hematopoietic SCT
Bone Marrow Transplantation (2011)
-
Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients
Bone Marrow Transplantation (2001)
-
Tacrolimus clearance is age-dependent within the pediatric population
Bone Marrow Transplantation (2000)